Abstract
Forkhead Box O transcription factors play important roles in bone metabolism by defending against oxidative stress and apoptosis. FoxO3a is of special interest as it is the predominant isoform expressed in bone. In osteoblasts, the administration of 1,25 dihydroxyvitamin D3 (1,25D3) increases FoxO3a expression, and alters calcium handling. We therefore queried whether FoxO3a participates in vitamin D-mediated regulation of calcium transport pathways or matrix calcification, independent of reactive oxygen species (ROS) formation. To examine this possibility, we differentiated MC3T3-E1 cells into mature osteoblast-like cells over 7 days. This coincided with an increased ability to mineralize extracellular matrix. FoxO3a expression increased throughout differentiation. 1,25D3 enhanced both FoxO3a mRNA and protein expression. Immunofluorescence microscopy found increased FoxO3a nuclear localization with differentiation and after treatment with 1,25D3. Live cell ratiometric imaging with Fura-2AM identified significant L-type calcium channel mediated calcium uptake that was enhanced by 1,25D3. We observed expression of both Cav1.2 and Cav1.3, although expression decreased throughout differentiation and was not altered by 1,25D3 treatment. FoxO3a overexpression reduced calcium uptake and calcium deposition. FoxO3a overexpression also prevented alterations in calcium channel expression and the cell differentiation associated decrease in expression of Runx2 and increased expression of osteocalcin, findings consistent with a failure for the cells to differentiate. Based on both our expression and functional data, we suggest that high levels of FoxO3a prevent osteoblast differentiation and matrix calcification.
Keywords: Forkhead Box O3, Osteoblast differentiation, Osteoblast mineralization, Matrix calcification, Calcium deposition, MC3T3-E1 cells
1. Introduction
Osteoporosis is a global health concern resulting in 8.9 million fractures every year and the incidence is expected to increase significantly with the rapidly aging population (Johnell and Kanis, 2006). Osteoporotic fractures significantly affect quality of life, and can be life-threatening especially in elderly people (Kanis et al., 2004). Osteoporosis results from an imbalance between bone resorption and subsequent bone formation by osteoblast cells during bone tissue remodeling. Osteoblasts originate from pluripotential mesenchymal cells and are capable of synthesizing new bone matrix during the bone formation phase of the remodeling cycle (Kartsogiannis and Ng, 2004). During the initial development stage in bone formation, osteoblast precursor cells first undergo proliferation, followed by osteoblast differentiation and then subsequently facilitate mineralization of the extracellular matrix (Quarles et al., 1992). Transcription factor Runx2 and glycoprotein osteocalcin (OCN) participate in osteoblast growth and differentiation. Runx2 has been described as the osteogenic “master switch” essential in turning on expression of subsequent biomarkers of mature osteoblasts, such as OCN. Runx2 is up-regulated in early stages of osteoblast differentiation, then down-regulated in mature osteoblasts (Komori, 2017). Osteocalcin is the most abundant noncollagenous protein secreted by osteoblasts. OCN expression is regulated by Runx2 expression and expression of OCN peaks during the mineralization phase of osteoblast activity (Stein et al., 1996).
The biologically active form of vitamin D (1,25 dihydroxyvitamin D3 [1,25D3]) augments bone mineralization via direct stimulation of osteoblast activity and indirectly through increasing intestinal calcium absorption (Van de Peppel and van Leeuwen, 2014). Upon binding to the vitamin D receptor (VDR), which is present in osteoblasts, 1,25D3 regulates expression of genes involved in osteoblast differentiation and mineralization, including alkaline phosphatase (ALP), osteocalcin (OCN), also known as bone gamma-carboxyglutamic acid-containing protein (BGLAP) and secreted phosphoprotein 1 (SPP1), also known as osteopontin (OPN) (Anderson, 1995; Van de Peppel and van Leeuwen, 2014; Woeckel et al., 2010).
The Forkhead box O (FoxO) transcription factors are a subclass of the large family of forkhead proteins, characterized by the winged-helix DNA binding domain known as the Forkhead box (Almeida, 2011; Calnan and Brunet, 2008). Mammals express four members of the FoxO family, FoxO1 (or FKHR), FoxO3 (FKHRL1), FoxO4 (AFX) and FoxO6 (Greer and Brunet, 2005). FoxO 1, 3 and 4 are expressed in bone while FoxO6 is predominantly expressed in brain (Jacobs et al., 2003). FoxOs participate in conserved signaling pathways and regulate transcriptional responses via tightly controlled post-translational modifications and alterations in their cellular localization (Almeida, 2011; Calnan and Brunet, 2008; Eelen et al., 2013). FoxOs are essential to translating environmental stimuli, including hormonal signals, inflammation and oxidative stress into dynamic gene expression necessary for cell proliferation, differentiation, apoptosis and stress resistance (Almeida, 2011).
Extensive research has been undertaken on the protective role of FoxOs in reducing age-related pathologies, including osteoporosis (Almeida, 2011). It was noted that a reduction in bone mass and strength in aging individuals was associated with an increasing incidence of bone fractures in those who exhibited high levels of oxidative stress (Almeida, 2011). Recent studies investigating the molecular mechanisms governing skeletal aging indicate that FoxOs mediate responses to oxidative stress by up-regulating antioxidant enzymes in addition to genes that control the cell cycle, DNA repair and life span thereby maintaining skeletal health (Almeida, 2011; Salih and Brunet, 2008; Van der Horst and Burgering, 2007). Knock-down of FoxOs in the murine pre-osteoblast MC3T3-E1 cell line led to a 3-fold increase in 1,25D3 induced ROS, without altering the antiproliferative action of 1,25D3 (Eelen et al., 2013). Consistent with that observation, conditional deletion of FoxO1, -3, and -4 from mature osteoblasts of adult mice led to increased oxidative stress and osteoblast apoptosis (Ambrogini et al., 2010). Those animals also displayed a reduced rate of bone formation and lower bone mass of cancellous and cortical bone, which the authors attributed to the role of FoxOs in protecting against oxidative stress (Ambrogini et al., 2010). In contrast, the deletion of FoxO1, -3 and -4 from osteoblast progenitor cells resulted in increased osteoblast number, mineralizing perimeter, mineral apposition rate, and ultimately higher bone mass (Iyer et al., 2013). In that model, the expression of FoxO-regulated antioxidant genes and apoptosis factors remained unaltered. Moreover, antioxidant levels were unchanged, suggesting that FoxOs might play a different role in osteoblast progenitor cells or osteoblast differentiation, other than protecting from ROS induced stress.
Despite extensive research into the role of FoxOs in maintaining bone health and regulation by the calciotropic hormone 1,25D3 the function of FoxOs at specific osteoblast-differentiation stages remains largely unknown. A possible role for FoxOs in the maintenance of calcium homeostasis in osteoblasts also remains unexplored. We therefore examined calcium homeostasis during stage-specific osteoblast differentiation in MC3T3-E1 cells. We found an L-type calcium channel to be the major functioning channel responsible for the majority of calcium influx in differentiated MC3T3-E1 osteoblast like cells. FoxO3a expression also increased throughout development. Administration of 1,25D3 further enhanced calcium influx and FoxO3a expression. To examine the role of FoxO3a in osteoblast differentiation we generated a stable MC3T3-E1 cell line over-expressing FoxO3a, and noted FoxO3a to be a negative regulator of osteoblast differentiation. Subsequently, high levels of FoxO3a reduced calcium influx through the L-type calcium channel and calcium deposition, consistent with an osteoporotic phenotype.
2. Material and methods
2.1. Cell culture
MC3T3-E1 cells are murine pre-osteoblast cells that were purchased from ATCC. They were cultured in α-MEM culture medium (Gibco Life Technologies, MA, USA) supplemented with 10% fetal calf serum (FBS) (VWR International, Canada) and 1% Penicillin-Streptomycin-Glutamine (PSG) (Gibco Life Technologies, MA, USA). Pre-osteoblasts underwent differentiation to osteoblasts by adding 50 mg/mL ascorbic acid and 10 mM β-glycerophosphate (Sigma-Aldrich, MO, USA) to the culture media. RNA and protein lysates were obtained from cell culture after incubation for 1 day, 3 days or 7 days.
2.2. Quantitative real-time PCR
Total mRNA was isolated with Trizol Reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, USA). After isolation, mRNA was first treated with DNaseI (Invitrogen, Carlsbad, USA) then 1 μg of RNA was reverse transcribed by Random Primers (Invitrogen, Carlsbad, USA) and SuperScript II reverse transcriptase (Invitrogen, Carlsbad, USA) as previously published (Pan et al., 2012). cDNA was used to determine FoxO3a, RXRα, VDR, Runx2, OCN (bglap), L-type calcium channel Cav1.2 (cacna1c), Cav1.3 (cacna1d), T-type calcium channel Cav3.1 (cacna1g), calbindin-D9K (S100 g), the plasma membrane Ca2+-ATPase (PMCA1b), the sodium/calcium exchanger, member 1 (NCX1, Slc8a1) mRNA expression. The housekeeping gene, 18S ribosomal RNA levels were used as an internal control and data normalized to 18S expression. Primers and probes used to evaluate gene expression are listed in Table 1.
Table 1.
Primers and probe sequences used for quantitative real-time PCR.
| FoxO3a | Forward: CGTTGTTGGTTTGAATGTGGG |
| Reverse: GGTTTTCTCTGTAGGTCTTCCG | |
| Probe: TGCCCATTTCCCCTTTCCTCAGT | |
| RXRα | Forward: GCCCAAGACTGAGACATACG |
| Reverse: AGCTCAGAAAAGTGTGGGATC | |
| Probe: AGCTCACCAAATGACCCTGTTACCAA | |
| VDR | Forward: GTCAGTTACAGCATCCAAAAGG |
| Reverse: AGGTAAAAGACTGGTTGGAGC | |
| Probe: TGGCACTTGACTTAAGCAGGACAATCT | |
| Txnip | Forward: ACATTATCTCAGGGACTTGCG |
| Reverse: AAGGATGACTTTCTTGGAGCC | |
| Probe: TTTGAGGATGTTGCAGCCCAGGA | |
| Runx2 | Forward: GCTATTAAAGTGACAGTGGACGG |
| Reverse: GGCGATCAGAGAACAAACTAGG | |
| Probe: CGGGAAACCAAGAAGGCACAGACA | |
| OCN (bglap) | Forward: CACCTAGCAGACACCATGAG |
| Reverse: GTTCACTACCTTATTGCCCTCC | |
| Probe: ACCTCACAGATGCCAAGCCCA | |
| Cav1.2 | Forward: AGCGACAAAAGGATCAAGGG |
| Reverse: GGGAATGTGGTAGGAGAATGG | |
| Probe: CATTGGCAGTGGCAGGVTTGAG | |
| Cav1.3 | Forward: AGTCAACCAGATAGCCAACAG |
| Reverse: TCCTCTTCCTCTTCACCTACTG | |
| Probe: CCCTTACCCGCCCTGTGATGT | |
| Cav3.1 | Forward: TGGTGACAACTGGAATGGTATTA |
| Reverse: CACGAAGTAGATGGGTGAGATG | |
| Probe: ACGGTGTTGTAGCAGGTGGACTC | |
| Calbindin-D9K (S100 g) | Forward: TGGATAAGAATGGCGATGGAG |
| Reverse: GCTAGAGCTTCAGGATTGGAG | |
| Probe: ACAGCACCTACTGATTGAACGCACG | |
| PMCA1b | Forward: CGCCATCTTCTGCACCATT |
| Reverse: CAGCCATTGCTCTATTGAAAGTTC | |
| Probe: CAGCTGAAAGGCTTCCCGCCAAA | |
| NCX (slc8a1) | Forward: TGGTCTGAAAGATTCCGTGAC |
| Reverse: AGTGACATTGCCTATAGACGC | |
| Probe: AGCTACCCAGGACCAGTATGCAGA | |
| 18S | Forward: GAGACTCTGGCATGCTAACTAG |
| Reverse: GGACATCTAAGGGCATCACAG | |
| Probe: TGCTCAATCTCGGGTGGCTGAA |
2.3. Immunoblotting
Protein was extracted from MC3T3-E1 cells and immunoblotting performed as previously described (Pan et al., 2012). Cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris Base, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 0.1% SDS, 1% NP-40, pH = 7.4) with 1% protease inhibitor (Calbiochem, CA, USA) and phenylmethane sulfonyl fluoride (PMSF) (Thermo Fisher Scientific, MA, USA) freshly added on the day of the experiment (Calbiochem, Gibbstown, NJ, USA). The harvested protein was incubated on ice for 5 min before centrifuging at 13,000 rpm for 5 min at 4 °C. Total protein concentration was determined with a Nanodrop 2000C Spectrophotometer (Thermo Fisher Scientific, MA, USA) using a bovine serum albumin (BSA) standard curve (Sigma-Aldrich, MO, USA). The expression of FoxO3a, RXRα, VDR and myc was assessed by Western blotting with anti-FoxO3a (D19A7) rabbit mAb (Cell Signaling, MA, USA), anti-retinoid receptor alpha (RXRα) (Abcam, MA, USA), anti-vitamin D receptor (VDR) (Abcam, MA, USA) and anti-c-Myc (Y69) rabbit mAb (Cell Signaling, MA, USA). For internal control, blots were stripped and blotted for β-actin (Santa Cruz Biotechnology, Santa Cruz, CA). Quantification of relative band intensity was performed with Image J Software and Image Lab™ software (Biorad, CA, USA).
2.4. Immunofluorescence
Visualization of FoxO3a expression in 7-day differentiated MC3T3-E1 cells was accomplished using an immunofluorescence protocol similar to one previously described (Dimke et al., 2013). In brief, MC3T3-E1 cells seeded on 25 mm glass coverslips were fixed with 4% paraformaldehyde (PFA) purchased from Canemco Inc. (QC, Canada) followed by 5% glycine quenching (Sigma-Aldrich, MO, USA). Cells were incubated with anti-FoxO3a (D19A7) rabbit mAb (Cell Signaling, MA, USA) for an hour at RT in a buffer containing: 5% milk and 0.2% TritonX-100 (Thermo Fisher Scientific, MA, USA) in phosphate buffer saline, pH 7.4. Cells were then incubated with a secondary donkey anti-rabbit monoclonal antibody conjugated to Cy3 (Jackson ImmunoResearch Laboratories Inc., PA, USA). Concurrently, the actin cytoskeleton was stained with Alexa Fluor-488 conjugated phalloidin (Invitrogen Molecular probes, CA, USA) and the nucleus stained with 4′,6′-diamidino-2-phenylindole, dihydrochloride (DAPI) (Invitrogen Molecular probes, CA, USA). Coverslips were mounted on a microscope slide with DAKO (VWR, PA, USA) and then visualized with a fluorescent Leica DMI6000 microscope equipped with an Olympus IX-81 inverted stand employing a 60× objective. Images were obtained with a EMCCD camera (Hamamatsu, Japan) driven by velocity 5.0.3 software. Pearson's correlation coefficient was determined with the same software on whole fields of cells.
2.5. Calcium flux studies
The ratiometric fluorescent dye Fura-2AM (Molecular Probes, OR, USA) was employed to determine intracellular calcium changes in MC3T3-E1 cells. Pre-osteoblast MC3T3-E1 cells were seeded onto 25 mm coverslips and underwent cell differentiation with ascorbic acid and β-glycerophosphate for 7 days. These 7-day differentiated MC3T3-E1 cells were then incubated with Fura-2AM for 30 min protected from light at room temperature. After washing, the coverslip was mounted on the stage of a fluorescent Leica DMI6000 microscope. The cells were subjected to excitation at 340 and then 380 nm and emission captured at 510 nm using the ET – Fura-2 filter set and a Flash 4.0 camera from Hamamatsu, (Hamamatsu, Japan). The imaging protocol utilized was adopted from Miederer and colleagues with minor modifications (Miederer et al., 2015). Cells were first perfused in 0.5 mM CaCl2 with 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 5 mM Hepes pH = 7.4 for 5 min until a stable signal was reached, then the external calcium concentration was raised to 3.0 mM CaCl2 with 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 5 mM Hepes pH = 7.4 for 5 min. Next the cells were switched to a nominally Ca2+-free buffer containing140 mM NaCl, 3 mM KCl, 3 mM MgCl2, 5 mM Hepes, 1 mM EGTA pH = 7.4 for 5 min before switching back to the first buffer of 0.5 mM CaCl2. Calcium uptake was measured in the first 30 s after cells were switched back to 0.5 mM CaCl2 and calcium channel antagonists were applied during this step to determine specific Ca2+ channel activity. Finally, the Grynkiewicz equation was employed to calculate an absolute [Ca2+] from the emission achieved by 340/380 excitation (Grynkiewicz et al., 1985) and the rate and magnitude change was normalized to the control condition. The calcium channel antagonists utilized included: Lanthanum chloride (LaCl3) as a non-specific cationic blocker, Ruthenium Red as a TRPV channel antagonist, and Felodipine as a L-type calcium channel antagonist (Hattori et al., 2012; Hoenderop et al., 2001; Paradis et al., 1974). (1S,2S)-2-(2-(N-[(3-Benzimidazol-2-yl)propyl]-N-methylamino)ethyl)-6-fluoro-1,2,3,4-tetrahydro-1-isopropyl-2-naphtyl cyclopropanecarboxylate dihydrochloride hydrate, NNC 55-0396 hydrate was employed as a T-type Cav3.1 calcium channel antagonist (Huang et al., 2015).
2.6. Calcium deposition assays
The ability of MC3T3-E1 cells to deposit calcium in vitro was assessed by Alizarin red staining (ARS) (Sigma-Aldrich, MO, USA) as others have done previously (Gregory et al., 2004). Cells were seeded in 6-well plates (Sigma-Aldrich, MO, USA) and then underwent cell differentiation as above. Cell layers were washed three times with 1× PBS (Sigma-Aldrich, MO, USA) followed by fixation for 15 min with 4% formaldehyde at room temperature (Sigma-Aldrich, MO, USA). Cells were then stained with 40 mM ARS for 30 min at room temperature. After staining, the dye was removed and the cells were then washed 5 times with deionized water. Next, 800 μL of 10% acetic acid (Sigma-Aldrich, MO, USA) was added to each well and incubated for 30 min at room temperature. Stained cell layers were then scraped and heated at 85 °C for 10 min and then centrifuged at 20,000g for 15 min. 10% ammonium hydroxide was added to neutralize the acid and absorbance was read at 405 nm along with 8 serial dilution standards. ARS quantification was calculated from the standard curve. The data were then presented as the concentration of calcium extracted in a fixed volume as a fraction of the control condition.
2.7. Generation and characterization of FoxO3a over-expressing MC3T3-E1 cells
Pre-osteoblast MC3T3-E1 cells were stably transfected with a pCMV6-Entry vector harboring FoxO3a cDNA with a C-terminal Myc-DDK tag engineered at the 3′ end of the gene which was purchased from Origene (MD, USA). Cells were transfected with Fugene 6 as we have with other cell lines previously (Pan et al., 2012). Stable cell lines were selected in the presence of 500 μM Geneticin (G418) (Thermo Fisher Scientific, MA, USA) and screened by immunoblotting and immunofluorescence of Myc-tagged FoxO3a.
2.8. Statistical analysis
The results are presented as the mean ± standard error of the sample group. Statistical analysis was completed with GraphPad Prism Version 7 (GraphPad software Inc., La Jolla, CA). For comparisons between two groups unpaired two-tailed t-tests were employed and for multiple group comparison analysis, a One-way ANOVA followed by Tukey multiple comparisons test was performed. A significance difference between groups was determined a priori to be a p-value < 0.05.
3. Results
3.1. FoxO3a expression increases in MC3T3-E1 cells as they undergo differentiation
We first sought to identify a model system that could be employed to examine the role of FoxO3a in the ability of osteoblasts to calcify extracellular matrix. When exposed to ascorbic acid and β-glycerophosphate, MC3T3-E1 cells exhibit a developmental sequence similar to osteoblasts of bone tissue in vivo. They undergo proliferation from undifferentiated osteoblast precursors, through pre-osteoblasts to osteoblasts that can form extracellular matrix and importantly mineralize this matrix (Quarles et al., 1992). Consistent with MC3T3-E1 cells differentiating into an osteoblast like cell the expression of Runx2, an early stage transcriptional regulator of osteoblast differentiation increased significantly after 1-day of differentiation (Fig. 1A). Further, Runx2 expression decreases significantly at 3 and 7 days post differentiation initiation, consistent with immature osteoblasts differentiating into mature differentiated osteoblasts (Fig. 1A).
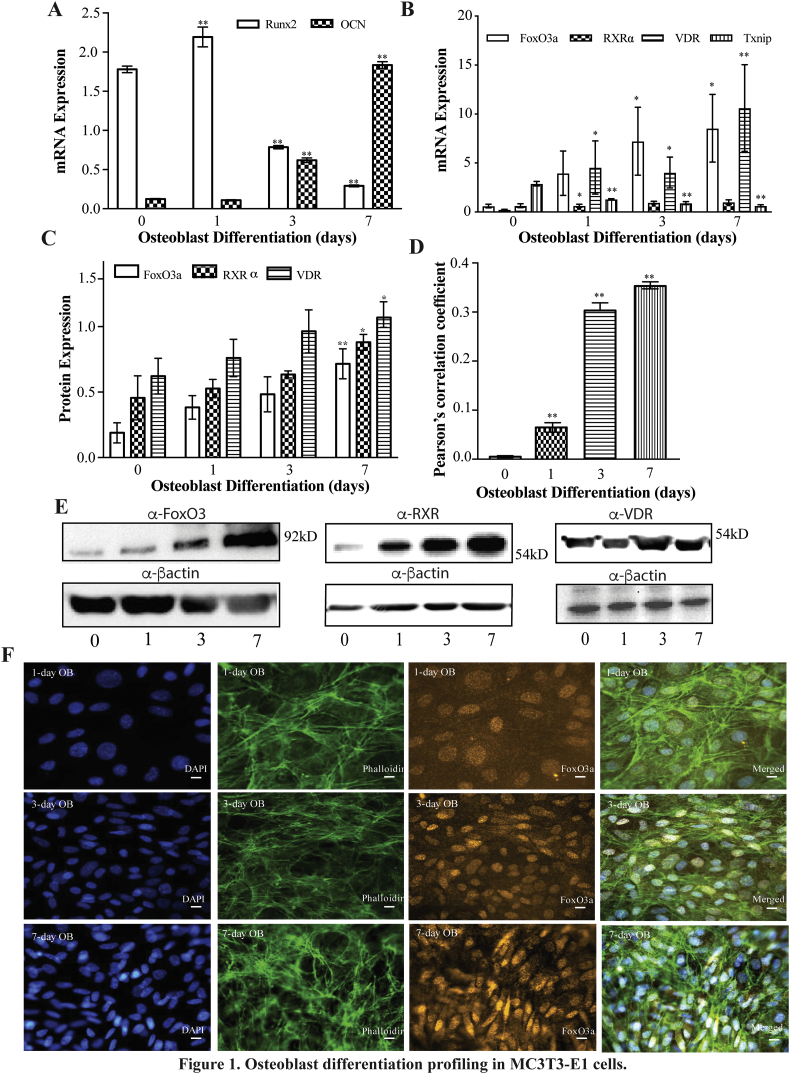
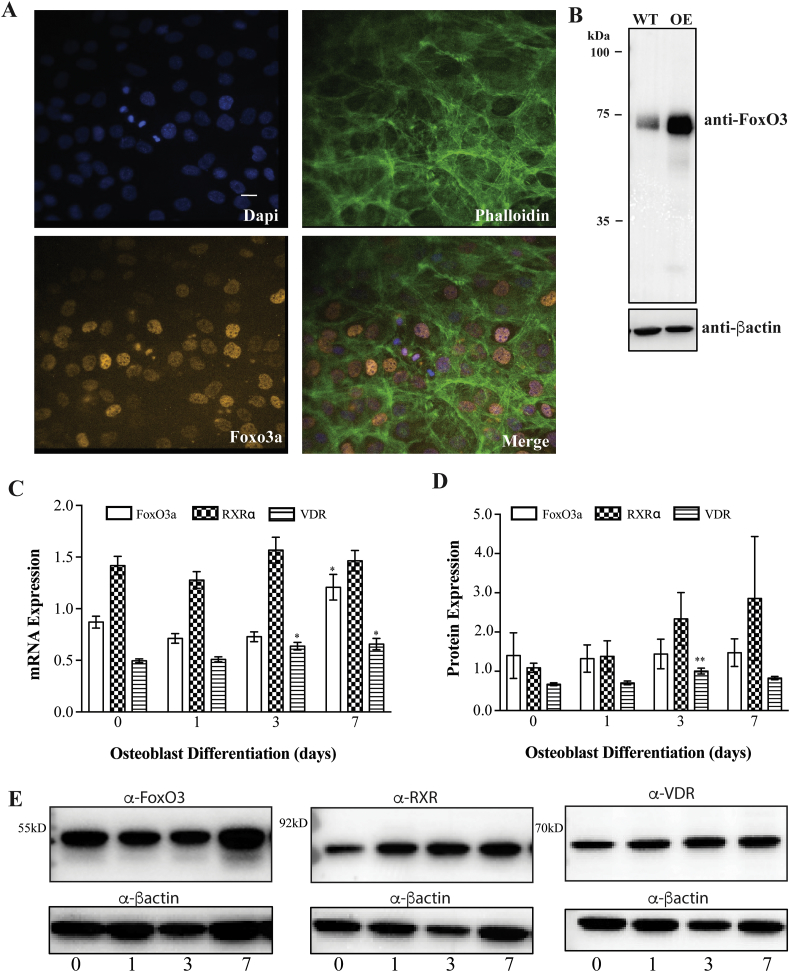
Fig. 1.
MC3T3-E1 cells can be differentiated into an osteoblast like cell line. A) Relative Runx2 and OCN mRNA expression, normalized to 18S. B). FoxO3a, RXRα, Txnip and VDR mRNA expression, normalized to 18S. C) Protein expression throughout differentiation and D) Pearson's correlation coefficient for FoxO3a and DAPI colocalization. E) Representative immunoblots from C. F) Representative immunofluorescence images of FoxO3a (orange), DAPI (blue) and phalloidin (green) throughout differentiation. Scale bar = 8 μM. ** represents p < 0.01, n is at least 6 for mRNA and protein expression studies. N is at least 15 independent images from at least 3 slides per condition for immunofluorescence studies. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Osteocalcin, OCN, is a non-collagenous protein secreted by middle to late stage differentiated osteoblasts. We observed an increase in OCN expression as cells underwent differentiation, which reached the highest levels in 7-day differentiated MC3T3-E1 cells (Fig. 1A). This provides more evidence that MC3T3-E1 cells undergo differentiation into a mature differentiated osteoblasts like cell. Further, high levels of OCN in 7-day differentiated MC3T3-E1 cells are supportive of the cells having a higher calcium-binding affinity, and being able to calcify matrix in vitro.
We next examined FoxO3a expression during osteoblast differentiation by quantitative real time PCR and immunoblotting. FoxO3a mRNA and protein expression increased over the course of differentiation (Fig. 1C and D). We also examined the localization of FoxO3a over the course of differentiation. To this end, we immuno-labelled FoxO3a with a red fluorophore and stained the nucleus with DAPI and then assessed co-localization of the two fluorescent signals by determining Pearson's correlation coefficient (PCC) (Fig. 1B and E). The PCC between FoxO3a and DAPI increased throughout the course of differentiation and the immunofluorescence signal from FoxO3a immunostaining demonstrated the highest levels of nuclear FoxO3a accumulation in 7-day differentiated MC3T3-E1 cells (Fig. 1E). Consistent with FoxO3a mediating changes in gene expression, VDUP1 (also known as Txnip), a gene regulated by FoxO3a, decreased in expression as the cells underwent differentiation (Fig. 1B) (Zhuo et al., 2010). Finally, we examined the expression of the vitamin D receptor (VDR) and RXRα throughout differentiation and found that they both increased throughout differentiation although to a lesser extent than FoxO3a (Fig. 1C and D).
3.2. MC3T3-E1 cells take up calcium and calcify extracellular matrix
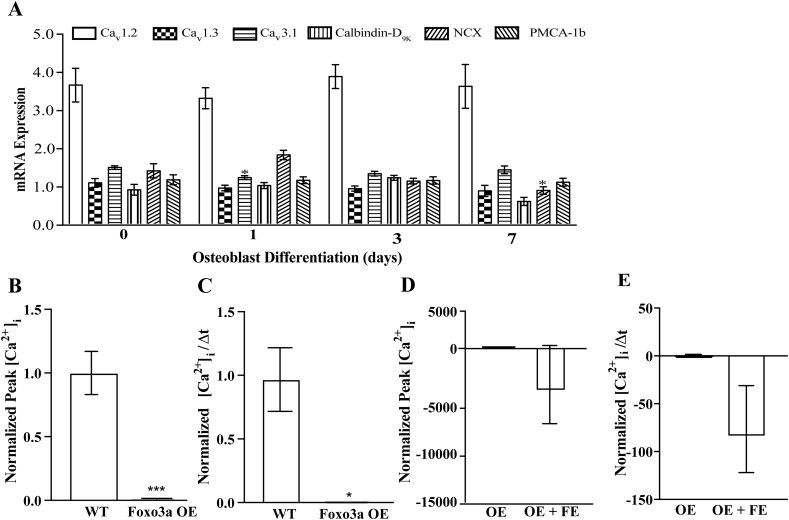
We next sought to identify potential molecules expressed by MC3T3-E1 cells that could participate in the calcification of extracellular matrix. We were able to detect mRNA from the L-type calcium channels Cav1.2 and Cav1.3, the T-type calcium channel Cav3.1, the calcium-binding protein, calbindin-D9K, the sodium calcium exchanger NCX and the plasma membrane calcium dependent ATPase PMCA-1b. We were unable to detect TrpV5, TrpV6 and calbindin-D28K mRNA. Interestingly, the expression of all these genes, which have been implicated in calcium transport across epithelia, decreased throughout cell differentiation (Fig. 2A).
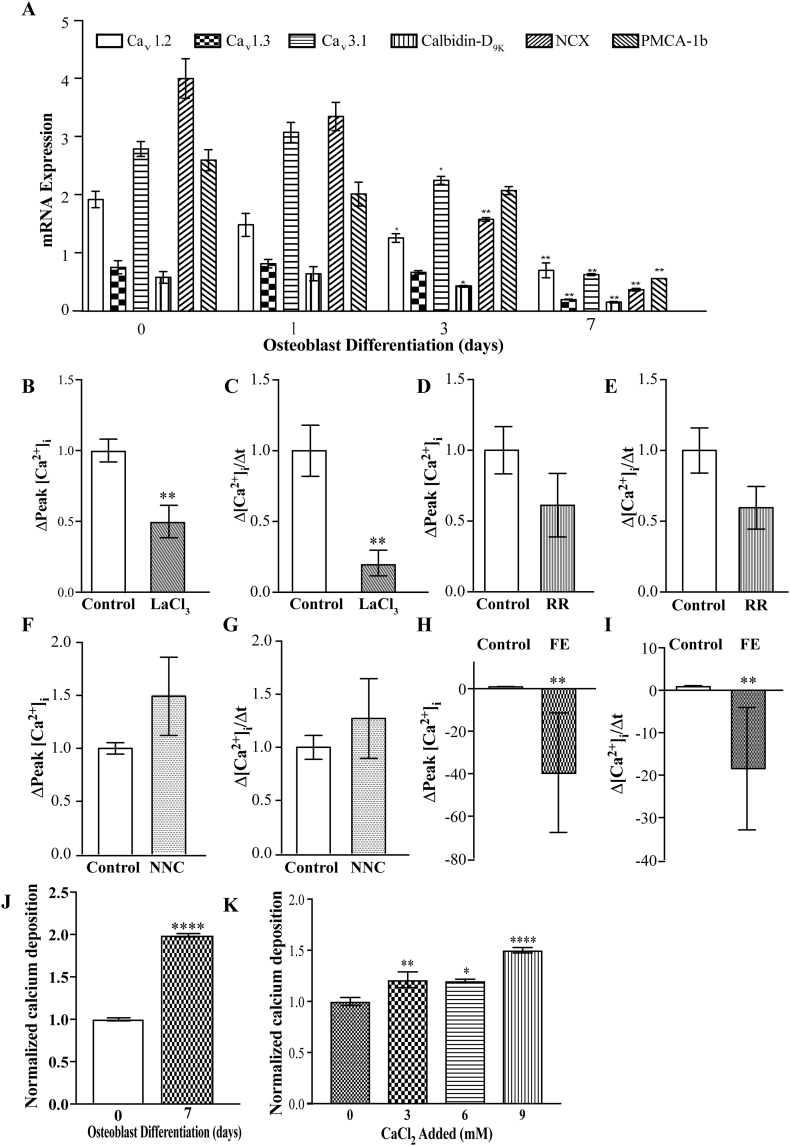
Fig. 2.
An L-type calcium channel mediates calcium uptake into 7-day differentiated MC3T3-E1 cells. A) mRNA expression of mediators of calcium fluxes throughout differentiation of MC3T3-E1 cells, normalized to 18S. B–I) Fura-2AM ratiometric live cell fluorescence imaging of single differentiated cells assessed as the magnitude of uptake, Δpeak (B, D, F and H) or rate (C, E, G and I) in the presence of either LaCl3 (B and C), ruthenium red (D and E), NNC (F and G) or Felodipine (H and I), normalized to the untreated condition. J) Calcium deposition by pre-osteoblasts (pOB) or 7 day differentiated MC3T3-E1 cells. K) Calcium deposition in 7-day differentiated cells in the presence of the amount of additional CaCl2 added to culture media, listed below the x-axis, data is per well and normalized to pOB in J and 0 CaCl2 added in K. * represents p < 0.05 and ** represents p < 0.01. For calcium flux studies, n is at least 6 separate coverslips, with at least 20 cells per coverslip analyzed per condition, scale bar = 8 μM. For calcium deposition studies n is >6 per condition.
To determine the functionality of these calcium transport genes we measured calcium uptake into 7-day differentiated MC3T3-E1 cells via live cell ratiometric calcium imaging with Fura-2AM. We used different calcium channel inhibitors to implicate specific channel activity in this process. First, 100 μM lanthanum chloride (LaCl3), a non-specific Ca2+ channel antagonist, reduce both the magnitude (Δpeak) and rate (Δslope) of calcium influx into MC3T3-E1 cells (Fig. 2B and C). Ruthenium red (RR), a TRPV channel antagonist had no significant effect on calcium uptake into MC3T3-E1 cells (Fig. 2D and E), consistent with our inability to detect Trpv5 and Trpv6 in this cell line. NNC 55-0396, a low-threshold (T) type Ca2+ channel antagonist also did not cause a significant alteration in either the magnitude nor rate of calcium uptake into MC3T3-E1 cells (Fig. 2F and G). However, 10 μM felodipine, an L-type - Ca2+ channel blocker, not only prevented calcium influx but its application resulted in significant calcium efflux from MC3T3-E1 cells (Fig. 2H and I). This is likely due to completely blocking influx mechanisms without altering efflux mechanisms. Together this data implies that the major mechanism by which calcium uptake occurs into differentiated MC3T3-E1 cells is through an L-type calcium channel (likely either Cav1.2 or Cav1.3).
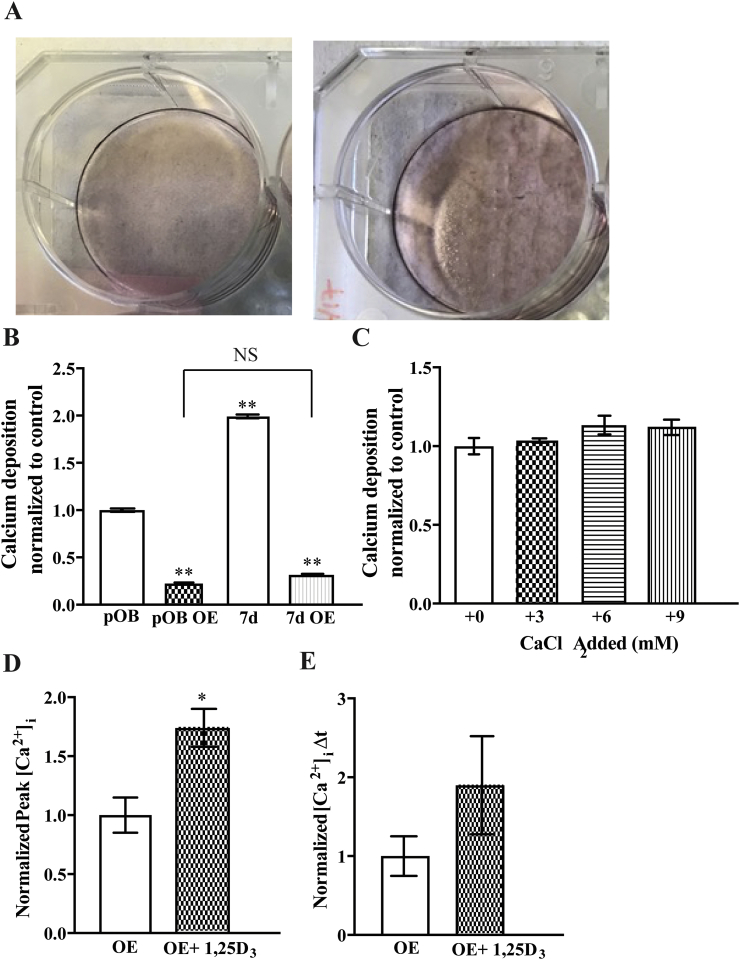
Having determined how calcium was being taken up into our osteoblast cell model we next examined the ability of these cells to mineralize matrix. Alizarin red staining was used to stain and quantify calcium deposited in the extracellular matrix. A significant increase in calcium deposition was observed after 7-days of M3T3-E1 cell differentiation (Fig. 2J). In order to assess whether matrix mineralization could be enhanced in our model system, additional calcium chloride (CaCl2) was added to 7-day differentiated cells. Incubation with an additional, 3–9 mM CaCl2 for 24 h increased the amount of calcium deposited in the matrix (Fig. 2K). Therefore, not only do MC3T3-E1 cells take up calcium, but they are able to deposit it in the extracellular matrix.
3.3. 1,25D3 increases FoxO3a expression, nuclear localization and calcium uptake
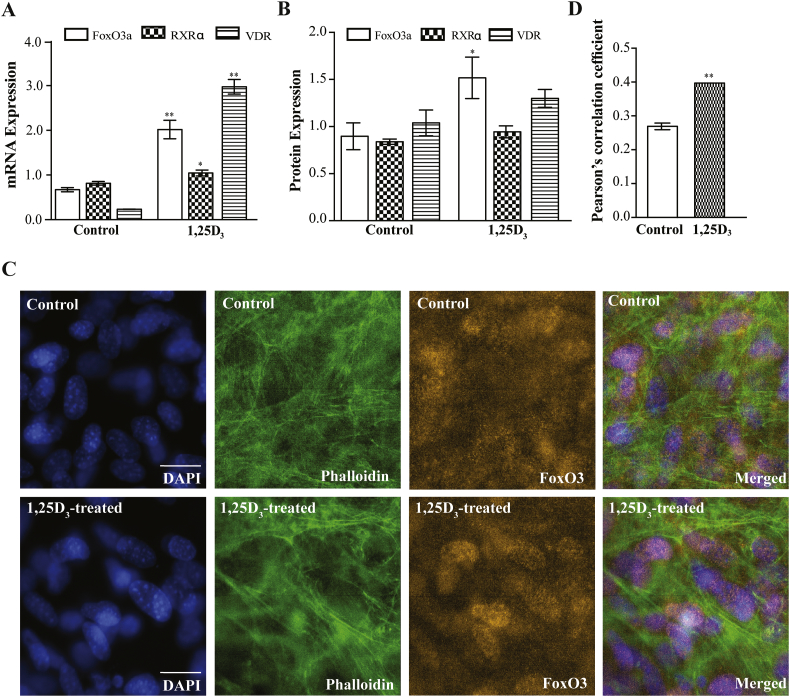
1,25-Dihydroxyvitamin D3 (1,25D3 i.e. calcitriol) has both direct and indirect effects on bone formation. In order to assess the direct effect of 1,25D3 on FoxO3a expression and nuclear localization in our cell culture model, FoxO3a expression and nuclear localization was determined as above on MC3T3-E1 cells treated with 100 nM 1,25D3 for 24 h. Treatment with 100 nM 1,25D3 increased FoxO3a mRNA and protein expression significantly in 7-day differentiated MC3T3-E1 cells (Fig. 3A, B and Supplemental material). This treatment also resulted in more FoxO3a being retained in the nucleus compared to control (Fig. 3C and D). Treatment with 1,25D3 also increased the expression of both RXRα and VDR at the level of mRNA but not protein (Fig. 3A, B and Supplemental material).
Fig. 3.
1,25D3 increases FoxO3a expression and nuclear localization in 7-day differentiated MC3T3-E1 cells. FoxO3a, RXRα and VDR A) mRNA expression normalized to 18S and B) protein expression, normalized to beta actin before and after 24 h treatment with 100 nM 1,25D3 after differentiation. Representative blots are presented in Supplemental materials. C) Representative immunofluorescence images of FoxO3a (orange), DAPI (blue) and phalloidin (green) before and after 1,25D3 treatment and D) Pearson's correlation coefficient for FoxO3a and DAPI colocalization before and after 1,25D3 treatment, scale bar = 20 μM. * represents p < 0.05 and ** represents p < 0.01, n is >6 per condition in A&B and at least 15 independent images from at least 3 slides per condition for the immunofluorescence studies. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
1,25D3 increases FoxO3a expression and nuclear localization in 7-day differentiated MC3T3-E1 cells. FoxO3a, RXRα and VDR A) mRNA expression normalized to 18S and B) protein expression, normalized to beta actin before and after 24 h treatment with 100 nM 1,25D3 after differentiation. Representative blots are presented in Supplemental materials. C) Representative immunofluorescence images of FoxO3a (orange), DAPI (blue) and phalloidin (green) before and after 1,25D3 treatment and D) Pearson's correlation coefficient for FoxO3a and DAPI colocalization before and after 1,25D3 treatment, scale bar = 20 μM. * represents p < 0.05 and ** represents p < 0.01, n is >6 per condition in A&B and at least 15 independent images from at least 3 slides per condition for the immunofluorescence studies. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
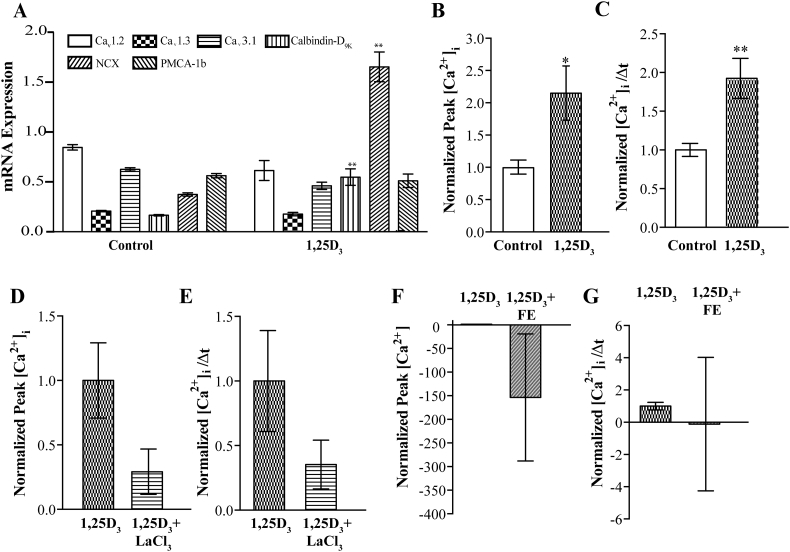
The effect of 1,25D3 on calcium uptake mechanisms in MC3T3-E1 cells was next examined. The mRNA expression of calcium channels and transporters was therefore determined after 7 day differentiated MC3T3-E1 cells were treated with 100 nm 1,25D3 for 24 h. 1,25D3 did not alter the expression of the L-type calcium channels Cav1.2, Cav1.3 nor the T-type Ca2+ channel Cav3.1 nor the plasma membrane calcium dependent ATPase, PMCA-1b, Fig. 4A. However, calbindin-D9K mRNA expression was up-regulated significantly by 1,25D3 as was NCX expression (Fig. 4A).
Fig. 4.
1,25D3 increases calcium influx in 7-day differentiated MC3T3-E1 cells via an L-type calcium channel. A) mRNA expression of mediators of calcium fluxes before and after 1,25D3 treatment, normalized to 18S B–G) Fura-2AM ratiometric live cell fluorescence imaging of differentiated cells assessed as the magnitude of uptake, Δpeak (B, D and F) or rate (C, E and G) in the presence of either 1,25D3 (B and C) or 1,25D3 and LaCl3 (D and E) or 1,25D3 and Felodipine (F and G), normalized to either untreated (B & C), or just 1,25D3 (D-G) condition. * represents p < 0.05 and ** represents p < 0.01, n is >6 per condition throughout, with 20 cells per coverslip analyzed per condition.
In order to examine the effect of 1,25D3 on calcium channel function, ratiometric live cell imaging with Fura-2AM was employed as above. We measured calcium uptake into 7-day differentiated MC3T3-E1 cells treated with 100 nM 1,25D3 or vehicle and found that 1,25D3 increased calcium influx (Fig. 4B and C). Treatment with LaCl3 attenuated the 1,25D3-mediated increase in calcium influx (Fig. 4D and E). In order to implicate L-type Ca2+ channels in this 1,25D3-mediated increase, 1,25D3-treated MC3T3-E1 cells were treated with 10 μM felodipine. This prevented the increase in calcium influx induced by 1,25D3 consistent with 1,25D3 increasing the activity of an L-type calcium channel (Fig. 4F and G).
3.4. Over-expression of FoxO3a prevents calcium influx into MC3T3-E1 cells
MC3T3-E1 cells differentiate into an osteoblast like cell line that demonstrates dynamic calcium influx and the ability to mineralize extracellular matrix. Coincident with this differentiation and ability to mineralize matrix, MC3T3-E1 cells increase FoxO3a expression and nuclear localization. Moreover, treatment with 1,25D3 enhances calcium uptake into the differentiated cells. Given the fundamental role of 1,25D3 in calcium homeostasis we hypothesized that 1,25D3 is mediating some of its effects through alterations in FoxO3a expression/activity. We were unable to successfully delete FoxO3a from MC3T3-E1 cells. Instead we over-expressed FoxO3a (Fig. 5A and B). 7-day differentiated MC3T3-E1 cells over-expressing FoxO3a demonstrated nearly exclusive nuclear localization of the transcription factor (Fig. 5A). Over-expression of FoxO3a increased baseline expression of RXRα and the increase in expression was seen throughout differentiation. In contrast, the overexpression of FoxO3a did not alter baseline expression of VDR nor did it prevent the increase in expression observed throughout development (Fig. 5C–E).
Fig. 5.
Characterization of MC3T3-E1 cells over-expressing FoxO3a. A) Representative immunofluorescence images of MC3T3-E1 cells over-expressing FoxO3a immunostained for FoxO3a (orange) DAPI (blue) or phalloidin (green), scale bar = 8 μM. B) Immunoblot of lysate form wild-type (WT) or cells over-expressing FoxO3a (OE), blotted for FoxO3a. FoxO3a, RXRα and VDR C) mRNA expression (normalized to 18S) and D and E) protein expression from MC3T3-E1 cells overexpressing FoxO3a throughout differentiation. * represents p < 0.05 and ** represents p < 0.01, n = 9 per condition for protein and mRNA quantification. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Examination of potential mediators of calcium flux into MC3T3-E1 cells overexpressing FoxO3a revealed that continued elevated expression of FoxO3a throughout differentiation prevented the decrease in Cav1.2, Cav1.3, Cav3.1, calbindin-D9K and PMCa1b but not NCX seen previously (Fig. 6A vs 2A). Interestingly, despite sustained increased levels of these calcium handling genes, ratiometric live cell calcium imaging on the FoxO3a over expressing cells demonstrated a dramatic reduction in calcium influx that was inhibited by Felodipine (Fig. 6B–E). This data demonstrates that an L-type calcium channel remains responsible for calcium influx in these cells and that its activity and/or expression is dramatically attenuated by FoxO3a.
Fig. 6.
FoxO3a over-expression attenuates L-type calcium channel mediated calcium influx into MC3T3-E1 cells. A) mRNA expression of mediators of calcium fluxes throughout differentiation in MC3T3-E1 cells over-expressing FoxO3a. Data normalized to 18S as an internal control. B–E) Fura-2AM ratiometric live cell fluorescence imaging of MC3T3-E1 wild-type (WT) or cells over-expressing (OE) FoxO3a assessed as the magnitude of uptake, Δpeak (B and D) or rate (C and E), normalized to the wild-type cell response. L-type calcium channel activity was assessed by the addition of Felodipine (FE), (D and E), n is >6 per condition throughout.
Given the significant attenuation in calcium influx induced by FoxO3a overexpression we examined the ability of these cells to mineralize matrix. In contrast to wild-type cells, the undifferentiated FoxO3a over-expressing cells had a similar ability to 7-day differentiated cells to mineralize matrix (Fig. 7). Further, at both differentiated stages (undifferentiated and 7-day differentiated) the overexpressing cells had significantly reduced matrix mineralization ability relative to wild-type cells (Fig. 7A and B). Empty vector transfected cells displayed the same ability to mineralize matrix as wildtype cells (2.1 ± 0.4 vs 1.9 ± 0.2 nmole calcium per well), which was statistically lower relative to the FoxO3a over-expressing cells (0.3 ± 0.1 nmol of calcium per well). Moreover, the over-expressing cells failed to increase matrix mineralization when incubated with additional extracellular calcium (Fig. 7C). The addition of 1,25D3 to the overexpressing cells resulted in a small but significant increase in calcium flux as per the wild-type cells, however, this was dramatically attenuated (Fig. 7D and E). Together these results suggest that overexpression of FoxO3a in undifferentiated MC3T3-E1 cells suppresses calcium uptake via an L-type calcium channel, suppresses matrix mineralization and attenuates the vitamin D mediated effects on calcium uptake.
Fig. 7.
FoxO3a over-expression in MC3T3-E1 cells attenuates matrix calcification. A) Representative images of MC3T3-E1 cells stained with Alizarin red pre (pOB) and 7 days post (7d) differentiation. Data from wildtype cells were included for comparison. B) Quantification of Alizarin red staining from A) and after addition of the amount of calcium chloride written below the x-axis C), data is per well and normalized to pOB in B and 0 CaCl2 added in C. D and E) Fura2 ratiometric live cell fluorescence imaging of MC3T3-E1 cells over-expressing (OE) FoxO3a assessed as the magnitude of uptake, Δpeak (D) or rate (E) in the absence and presence of 100 nM 1,25D3 for 24 h, normalized to the untreated condition. * represents p < 0.05 and ** represents p < 0.01, n is = 9 per condition throughout. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.5. FoxO3a over-expression prevents a decrease in expression of Runx2 and an increase in expression of OCN
The deletion of FoxO1, -3, and -4 from osteoblast progenitor cells increased osteoblast number, consistent with increased proliferation and differentiation (Iyer et al., 2013). Given this and the reduced matrix mineralization ability and lack of significant calcium influx into FoxO3a over-expressing cells, a phenotype resembling undifferentiated osteoblasts, we inquired whether FoxO3a over-expression might inhibit proliferation and differentiation. Consistent with this when plated in similar numbers, total protein harvested per well of 7 day differentiated osteoblasts was significantly reduced relative to wild-type cells (192 vs 42 μg/mL), consistent with decreased proliferation. However, the reduction in protein content was less than the ability to mineralize matrix, suggesting both reduced single cell ability to mineralize matrix and global inhibition of matrix mineralization.
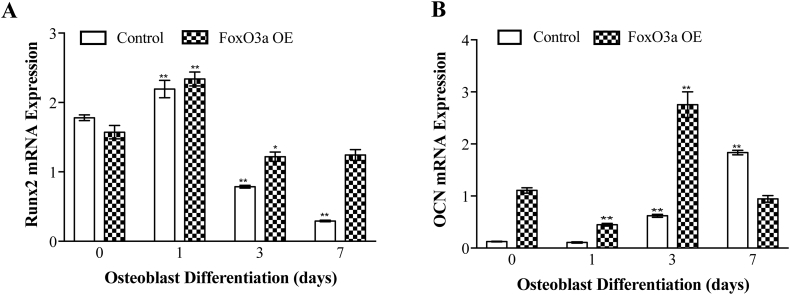
To examine differentiation, we assessed Runx2 and OCN expression in the over-expressing cells. We found that Runx2 failed to decrease over time in the FoxO3a overexpressing cells and after 7 days of differentiation was not different than the predifferentiated cells (Fig. 8A). Similarly, while there was an initial increase in OCN expression in the FoxO3a overexpressing MC3T3-E1 cells, expression of OCN after 7 days of differentiation was not different than before differentiation, in sharp contrast to the wild-type cells where OCN expression dramatically increased throughout development (Fig. 8B). Importantly this data was normalized to an internal control and should therefore not be affected by cell number. These findings infer that FoxO3a over-expression inhibits osteoblast differentiation.
Fig. 8.
FoxO3a over-expression inhibits osteoblast differentiation. A) Runx2 mRNA expression and B) OCN mRNA expression in MC3T3-E1 cells throughout differentiation. Control is wild-type cells. All data normalized to 18S. * represents p < 0.05 and ** represents p < 0.01, n = 9 per cell type.
4. Discussion
The FoxOs have emerged as critical regulators of proliferation, differentiation, metabolism and apoptosis (Eelen et al., 2013; Weigel et al., 1989). Research examining the functional role of FoxOs in bone homeostasis yielded different results in osteoblast progenitor cells, osteoblasts and osteoclasts (Almeida, 2011; Ambrogini et al., 2010; Bartell et al., 2014; Iyer et al., 2013). Iyer and colleagues hypothesized FoxOs play multi-functional roles during the 3 distinct stages of osteoblast differentiation including early progenitors, committed osteoblast precursors and osteoblasts (Iyer et al., 2013). Our data supports an additional role of FoxO3a in differentiating osteoblasts before they become mature osteoblasts. FoxO3a mRNA and protein expression gradually increases during differentiation with the majority of FoxO3a moving into the nucleus to regulate gene expression. FoxO3a can be further increased by treatment with vitamin D, a treatment that also augments calcium uptake. FoxO3a over-expression in this model system dramatically attenuated calcium uptake, matrix mineralization, and the response to vitamin D. This does not appear to be due to a direct effect on the calcium transporting capabilities of the cells but instead might be secondary to inhibiting appropriate differentiation of the preosteoblastic cells into mature osteoblasts.
We observed that in wild-type MC3T3-E1 cells undergoing differentiation, Runx2 expression peaks in immature osteoblasts followed by down-regulation in 3-day and 7-day differentiated osteoblasts. Minimal OCN expression was detected in pre-osteoblasts, which then increased significantly in 3-day osteoblasts reaching the highest levels in 7-day differentiated osteoblasts. These data support that MC3T3-E1 cells can differentiate in vitro, in a manner similar to events involved with in vivo bone formation. In contrast, high levels of FoxO3a expression in preosteoblasts disrupted Runx2 and OCN mRNA expression, consistent with them not differentiating into functional osteoblasts.
Calcium deposition measurements also demonstrated the overexpressing cells fail to deposit Ca2+ into the extracellular matrix, to the same extent as wild-type or empty vector transfected cells. Another way to tease out whether there is a direct role of FoxO3a on these pathways would be to delete FoxO3a in osteoblasts using CRISPR/Cas 9 technology. Since FoxO3a was found to be a negative regulator of osteoblast development and matrix mineralization, in a FoxO3a-deficient in vitro model, we predicted enhanced differentiation leading to augmented calcification of the extracellular matrix. Unfortunately, we were unable to study MC3T3-E1 cells in the absence of FoxO3a, as those cells grew at a significantly reduced rate, leading to the continued escape of the knockout. RXRα and VDR mRNA and protein expression also exhibited a similar trend suggesting as the cells differentiate they are more able to respond to vitamin D stimuli. FoxO3a mRNA and protein expression was first at low levels in pre-osteoblasts, which was then up-regulated as cells underwent osteoblast differentiation reaching the highest levels in 7-day differentiated MC3T3-E1 cells. Moreover, assessment of nuclear localization confirmed that FoxO3a is more abundant in the nucleus as they undergo differentiation, consistent with a transcriptional role in differentiated osteoblasts. The overexpression of FoxO3a prevented many of the changes in calcium transporting genes observed throughout development and attenuated the effect of vitamin D on calcium uptake. This is consistent with a direct effect of FoxO3a on regulating the expression and activity of the calcium transporting machinery (as dynamic changes in FoxO3a coincided with the dynamic changes in expression observed). However, it is less likely that this is the case as over-expression of FoxO3a prevented transcriptional changes consistent with delayed or arrested differentiation of the cell line, as assessed by the absence of typical alterations in Runx2 and OCN. Instead we propose that the inhibition of differentiation likely explains these observations.
In a study by Iyer and colleagues, targeted deletion of FoxOs in osteoblast progenitor cells did not alter expression of genes responding to antioxidant stress or apoptosis nor did they show increased levels of oxidative stress in bone, suggesting the effects of FoxOs in osteoblast progenitors are independent of ROS (Iyer et al., 2013). This is consistent with our data. In contrast, when FoxOs were deleted from mature osteoblasts, it resulted in an osteopenic phenotype with increased osteoblast apoptosis, decreased osteoblast number, thereby resulting in reduced bone mass (Ambrogini et al., 2010). In the same study, overexpression of a gain of function FoxO3a in differentiated osteoblasts led to increased bone mass. Importantly, these perturbations all had an effect on bone mass, though it was not dramatic. These combined data suggest regulation of FoxOs in bone is differentiation stage-specific, where FoxO3 protects against ROS damage in differentiated osteoblasts, yet inhibits the differentiation of preosteoblasts into mature osteoblasts. This is consistent with our work, where we find that over-expression of FoxO3a in undifferentiated cells leads to a phenotype consistent with delayed osteoblast differentiation into a mature osteoblast, and the resultant likely consequence of this; i.e. reduced calcium uptake and matrix mineralization capabilities.
In conclusion, we demonstrate that increased FoxO3a expression in a cell culture model attenuates L-type mediate calcium channel uptake and responses to vitamin D. Moreover, FoxO3a over-expression in preosteoblasts also attenuates cell growth and potentially differentiation resulting in reduced matrix mineralization. This might be due to a direct effect of FoxO3a on the expression of genes responsible for these processes or more likely is a general effect on the differentiation of preosteoblasts into mature osteoblasts.
The following are the supplementary data related to this article.
Disclosures
The authors have nothing to disclose.
Transparency document
Transparency document.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR, MOP 136891) to R. Todd Alexander who is the Canada Research Chair in Renal Epithelial Transport Physiology and a Distinguished Researcher of the Stollery Children's Hospital Foundation.
Footnotes
The Transparency document associated with this article can be found, in online version.
References
- Almeida M. Unraveling the role of FoxOs in bone-insights from mouse models. Bone. 2011;49(3):319–327. doi: 10.1016/j.bone.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini E., Almeida M., Martin-Millan M., Paik J.K., Depinho R.A., Han L., Goellner J., Weinstein R.S., Jilka R.L., O'Brien C.A., Manolagas S.C. FoxO-mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metab. 2010;11(2):136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995;(314):266–280. [PubMed] [Google Scholar]
- Bartell S.M., Kim H.N., Ambrogini E., Han L., Iyer S., Serra Ucer S., Rabinovitch P., Jilka R.L., Weinstein R.S., Zhao H., O'Brien C.A., Manolagas S.C., Almeida M. FoxO proteins restrain osteoclastogenesis and bone resorption by attenuating H2O2 accumulation. Nat. Commun. 2014;5:3773. doi: 10.1038/ncomms4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calnan D.R., Brunet A. The FoxO code. Oncogene. 2008;27(16):2276–2288. doi: 10.1038/onc.2008.21. [DOI] [PubMed] [Google Scholar]
- Dimke H., Desai P., Borovac J., Lau A., Pan W., Alexander R.T. Activation of the Ca(2+)-sensing receptor increases renal claudin-14 expression and urinary Ca(2+) excretion. Am. J. Physiol. Renal Physiol. 2013;304(6):F761–F769. doi: 10.1152/ajprenal.00263.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eelen G., Verlinden L., Meyer M.B., Gijsbers R., Pike J.W., Bouillon R., Verstuyf A. 1,25-Dihydroxyvitamin D3 and the aging-related Forkhead Box O and Sestrin proteins in osteoblasts. J. Steroid Biochem. Mol. Biol. 2013;136:112–119. doi: 10.1016/j.jsbmb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24(50):7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- Gregory C.A., Gunn W.G., Peister A., Prockop D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004;329(1):77–84. doi: 10.1016/j.ab.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R.Y. A new generation Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260(6):3440–3450. [PubMed] [Google Scholar]
- Hattori T., Ara T., Fujinami Y. Pharmacological evidence for the involvement of calcium entry through TRPV1 channels in nifedipine-indiced [Ca2+]i elevation in gingival fibroblasts. Pharmacol. Pharm. 2012;3:427–432. [Google Scholar]
- Hoenderop J.G.J., Vennekens R., Muller D., Prenen J., Droogmans G., Bindels R.J.M., Nilius B. Function and expression of the epithelial Ca2+ channel family: comparison of mammalian ECaC1 and 2. J. Physiol. 2001;537(Pt3):747–761. doi: 10.1111/j.1469-7793.2001.00747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Lu Cm Wu Y., Ouyang S., Chen Y. T-type calcium channel antagonists, mibefradil and NNC-55-0396 inhibit cell proliferation and induce cell apoptosis in leukemia cell lines. J. Exp. Clin. Cancer Res. 2015;34(1):54. doi: 10.1186/s13046-015-0171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer S., Ambrogini E., Bartell S.M., Han L., Roberson P.K., de Cabo R., Jilka R.L., Weinstein R.S., O'Brien C.A., Manolagas S.C., Almeida M. FOXOs attenuate bone formation by suppressing Wnt signaling. J. Clin. Invest. 2013;123(8):3409–3419. doi: 10.1172/JCI68049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs F.M.J., Van der Heide L.P., Wijchers P.J.E.C., Burbach J.P.H., Hoekman M.F.M., Smidt M.P. FoxO6, a novel member of the FoxO class of transcription factors with distinct shuttling dynamics. J. Biol. Chem. 2003;278(38):35959–35967. doi: 10.1074/jbc.M302804200. [DOI] [PubMed] [Google Scholar]
- Johnell O., Kanis J.A. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos. Int. 2006;17(12):1726–1733. doi: 10.1007/s00198-006-0172-4. [DOI] [PubMed] [Google Scholar]
- Kanis J.A., Johnell O., De Laet C., Johansson H., Oden A., Delmas P., Eisman J., Fujiwara S., Garnero P., Kroger H., McCloskey E.V., Mellstrom D., Melton L.J., Pols H., Reeve J., Silman A., Tenehouse A. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35(2):375–382. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Kartsogiannis V., Ng K.W. Cell lines and primary cell cultures in the study of bone cell biology. Mol. Cell. Endocrinol. 2004;228(1–2):79–102. doi: 10.1016/j.mce.2003.06.002. [DOI] [PubMed] [Google Scholar]
- Komori T. The functions of Runx family transcription factors and Cbfb in skeletal development. Adv. Exp. Med. Biol. 2017;962:83–93. doi: 10.1007/978-981-10-3233-2_6. [DOI] [PubMed] [Google Scholar]
- Miederer A.M., Alansary D., Schwar G., Lee P.H., Jung M., Helms V., Niemeyer B.A. A STIM2 splice variant negatively regulates store-operated calcium entry. Nat. Commun. 2015;6:6899. doi: 10.1038/ncomms7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W., Borovac J., Spicer Z., Hoenderop J.G., Bindels R.J., Shull G.E., Doschak M.R., Cordat E., Alexander R.T. The epithelial sodium/proton exchanger, NHE3, is necessary for renal and intestinal calcium (re)absorption. Am. J. Physiol. Renal Physiol. 2012;302(8):F943–F956. doi: 10.1152/ajprenal.00504.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradis G.R., Bassingthwaighte J.B., Kelly P.J. Inhibition of transport of 47Ca and 85Sr by lanthanum in canine cortical bone. J. Appl. Physiol. 1974;36(2):221–225. doi: 10.1152/jappl.1974.36.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarles L.D., Yohay D.A., Lever L.W., Caton R., Wenstrup R.J. Distinct proliferative and differentiated stages of murine MC3T3-E1 cells in culture: an in vitro model of osteoblast development. J. Bone Miner. Res. 1992;7(6):683–692. doi: 10.1002/jbmr.5650070613. [DOI] [PubMed] [Google Scholar]
- Salih D.A., Brunet A. FoxO transcription factors in the maintenance of cellular homeostasis during aging. Curr. Opin. Cell Biol. 2008;20(2):126–136. doi: 10.1016/j.ceb.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein G.S., Lian J.B., Stein J.L., Van Wijinen A.J., Montecino M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996;76(2):593–629. doi: 10.1152/physrev.1996.76.2.593. [DOI] [PubMed] [Google Scholar]
- Van de Peppel J., van Leeuwen J.P.T.M. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014;5:137. doi: 10.3389/fphys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Horst A., Burgering B.M. Stressing the role of FoxO proteins in lifespan and disease. Nat. Rev. Mol. Cell Biol. 2007;8(6):440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jurgens G., Kuttner F., Seifert E., Jackle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Woeckel V.J., Alves R.D., Swagemakers S.M., Eijken M., Chiba J., van der Eerden B.C., van Leeuwen J.P. 1Alpha,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J. Cell. Physiol. 2010;225(2):593–600. doi: 10.1002/jcp.22244. [DOI] [PubMed] [Google Scholar]
- Zhuo D.X., Niu X.H., Chen Y.C., Xin D.Q., Guo Y.L., Mao Z. Bin. Vitamin D3 up-regulated protein 1(VDUP1) is regulated by FOXO3A and miR-17-5p at the transcriptional and post-transcriptional levels, respectively, in senescent fibroblasts. J. Biol. Chem. 2010;285(41):31491–31501. doi: 10.1074/jbc.M109.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Transparency document.