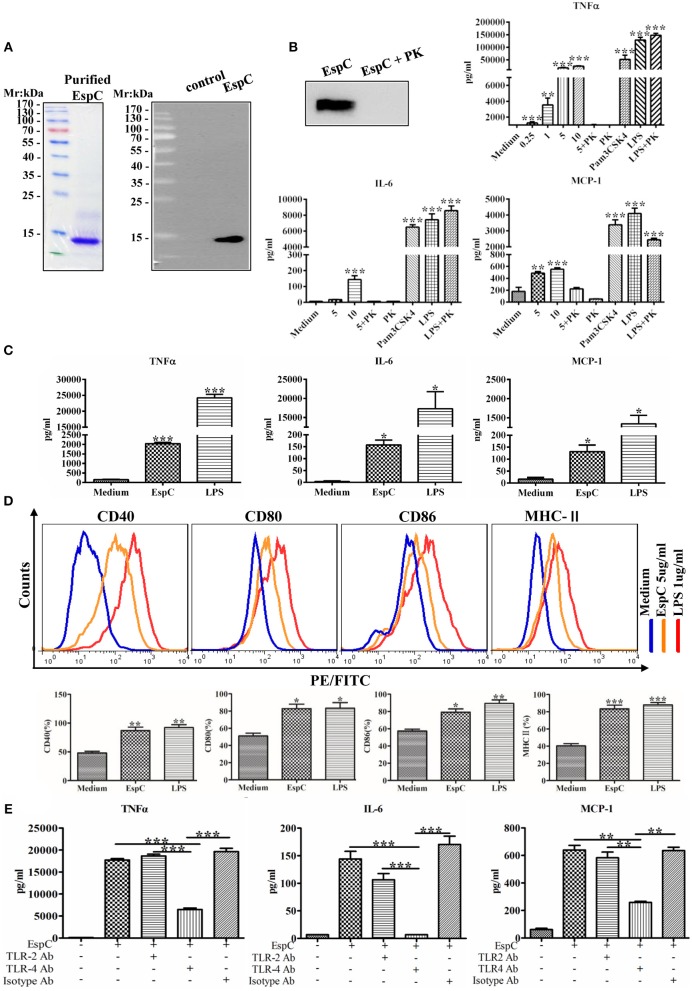
Figure 1.
EspC induced cytokine secretion and the production of costimulatory and MHC-II molecules in macrophages. (A) The protein was expressed in Escherichia coli and purified by Ni-NTA affinity chromatography. The purified protein was subjected to SDS-PAGE analysis by staining with Coomassie blue or to western blot analysis using anti-His mouse antibodies. (B) Purified EspC protein (5 μg/mL) was treated with or without proteinase K overnight at 56°C and was then subjected to western blot analysis using anti-His mouse antibodies. RAW264.7 cells were incubated with EspC (0.25, 1, 5, or 10 μg/mL) and EspC (5 μg/mL) treated with proteinase K, proteinase K alone, LPS (1 μg/mL), LPS (1 μg/mL) treated with proteinase K, or Pam3CSK4 (5 μg/mL). After incubation for 24 h, cell culture supernatants were collected, and TNF-α, IL-6, and MCP-1 levels were measured by ELISA. (C) THP-1 cells were differentiated in the presence of PMA for 48 h and then incubated with EspC (5 μg/mL) or LPS (1 μg/mL). After incubation for 36 h, cell culture supernatants were collected, and TNF-α, IL-6, and MCP-1 levels were measured by ELISA. (D) The expression levels of surface markers of RAW264.7 cells, including CD80, CD86, CD40, and MHC-II, were determined at 24 h after treatment with medium alone, 5 μg/mL EspC, or LPS by FACS analysis using respective FITC- or PE-conjugated monoclonal antibodies. (E) RAW264.7 cells were incubated with blocking antibodies before stimulation with EspC. TNF-α, IL-6, and MCP-1 production levels were measured by ELISA. All data are expressed as means ± SEMs (n = 3); *p < 0.05, **p < 0.01, ***p < 0.001.