Figure 4.
AMOT-p130 Control of YAP Localization in Response to the Actin Cytoskeleton
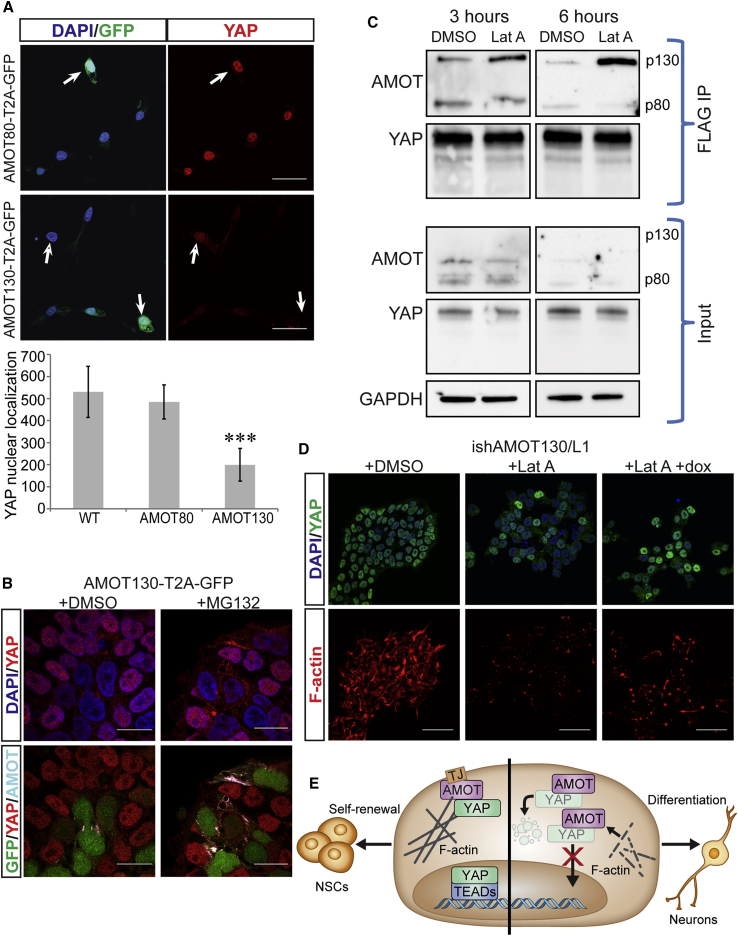
(A) Top panels: subcellular localization of YAP in AMOT130-T2A-GFP H9 cells (top) or AMOT80-T2A GFP H9 cells (bottom), 24 h after induction. Filled arrows indicate cells producing AMOT/GFP, and hollow arrows indicate cells that fail to produce AMOT/GFP. Bottom panel: quantification of YAP nuclear localization (n = 30 cells over three independent experiments); y axis is fluorescence intensity of YAP in the nucleus. Data are presented as mean ± SEM; ∗∗∗p < 0.0001 by Student's t test.
(B) Subcellular localization of YAP and AMOT following forced expression of AMOT-p130 and treatment with DMSO (left) or 1 h of 1 μM MG-132 (right).
(C) Top: immunoblots of FLAG coimmunoprecipitation (IP) after 3 and 6 h of DMSO or 1 μM lat-A treatment. Bottom: IP input.
(D) Subcellular localization of YAP (top) or F-actin staining (bottom) after treatment with DMSO (left), 6 h of 1 μM lat-A (middle), or doxycycline (dox) induction of AMOT-p130 and AMOTL1 shRNAs (right).
(E) Proposed mechanism for AMOT-mediated regulation of YAP during neural differentiation. In NSCs, AMOT sequesters YAP at TJ, and a pool of nuclear YAP remains where it regulates self-renewal of NSCs. During initiation of neuronal commitment, TJs are downregulated and AMOT expression further increases. In soft microenvironments, F-actin polymerization is reduced and AMOT liberated. These events enable AMOT to sequester YAP in the cytoplasm where YAP undergoes proteasomal degradation. An absence of YAP from the nucleus lifts repression of neuronal differentiation.
Scale bars, 50 μm. See also Figures S3 and S4.