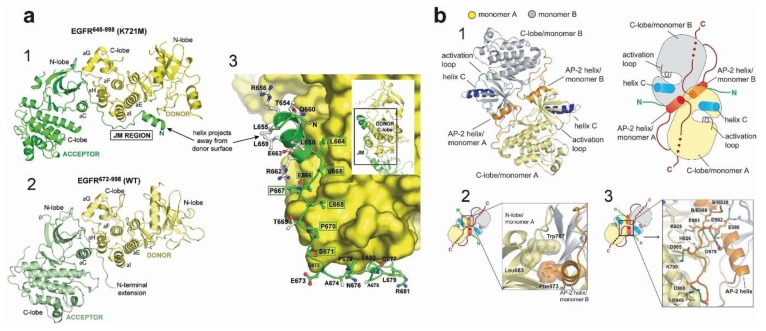
Figure 5.
Asymmetric and symmetric kinase dimers showing interactions with the juxtamembrane (JM) and C-terminal domains. (a) Crystal structure of the EGFR kinase from residue 645 to 998, including the entire inner JM region and some 40 residues of the C-terminus. The structure shows that the C-terminal half of the JM of the receiver cradles the C-lobe of the activator, thereby stabilising the asymmetric dimer. (1) shows the structure with an inactivating K721M mutation, closely resembling the structure for EGFR lacking the JM region (2). (3) shows the detail of the side-chains in the acceptor JM region (green), in contact with the C-lobe of the donor kinase domain. Taken, with permission, from Red-Brewer et al. [179]. (b) Structure of the symmetric inactive dimer. (1) gives an overview of the structure, while (2) shows the hydrophobic packing between the C-terminal AP-2 helix and the N-lobe of monomers A and B, respectively. (3) shows the electrostatic hook that forms between the C-terminal tail of EGFR and the hinge region of its kinase domain. Taken, with permission, from Jura et al. [177].