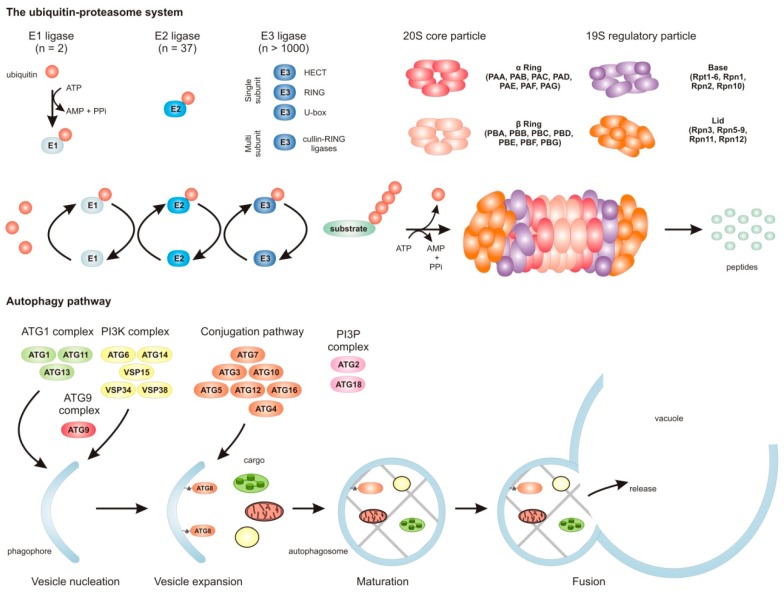
Figure 1.
Overview of the main cellular protein clearance mechanisms, the ubiquitin–proteasome system (UPS), and the autophagy pathway. Protein degradation by the UPS is initiated by the specific labeling of target proteins with ubiquitin. Attachment of a ubiquitin molecule requires the action of three enzymes, an ATP-dependent ubiquitin-activating enzyme (E1), a subsequent ubiquitin-conjugating enzyme (E2), and finally a ubiquitin ligase (E3) that transfers the ubiquitin from E2 to a target protein. After (poly)ubiquitination, the target protein is recognized and degraded by the 26S proteasome. Proteasomes contain a 19S regulatory particle and a catalytic active core particle (20S). The 19S regulatory particle recognizes the substrates, deubiquitinates the substrates, and unfolds them at the expense of ATP. The unfolded protein is translocated into the active chamber of the 20S particle to be degraded by the different proteases. The lower part of the figure represents the autophagy pathway. Autophagosomes are initiated by the formation of a phagophore at the outer surface of the ER through the action of the ATG1 complex, ATG9, and the PI3K complex. The growing phagophore surrounds cellular targets in either a selective or nonselective manner. Especially ATG8 plays a major role in substrate recognition for both the selective and nonselective pathways. A mature autophagosome moves toward the vacuole and fuses with it to release its cargo for proteolytic processing.