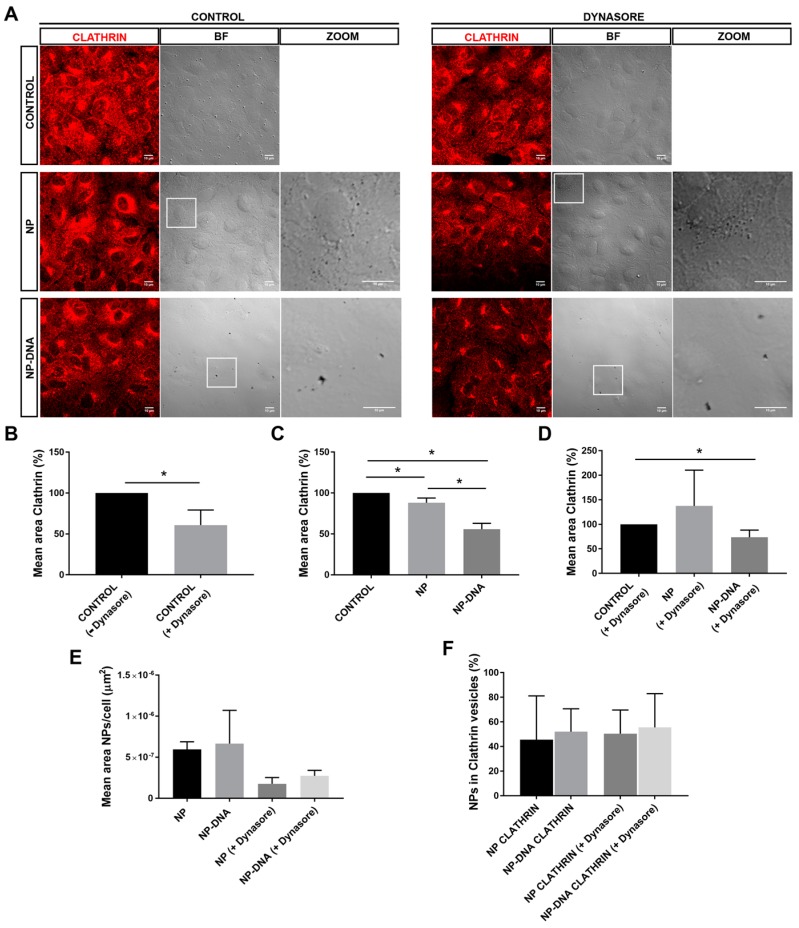
Figure 2.
DNA-gold nanoparticles are uptaken by differentiated ARPE-19 cells through different vesicular and/or non-vesicular routes. (A) After 2 h post-transfection, between 40 and 60% of pristine gold NPs and DNA-wrapped gold NPs are detected in clathrin-coated vesicles. NPs are visualized as black dots in the optic transmission microscopy channel (BF-bright field, see also zoom panels). DNA nanoparticles appear in clusters compared to pristine 40 nm gold particles. Clathrin-coated vesicles are immunodetected in red. Dynasore treatment inhibited the uptake by dynamin-mediated events. Image analyses of colocalization were performed by using masks over different channels. (B) Treatment with dynasore reduced down to 60% the number of clathrin-coated vesicles in untransfected cells, after 2 h. Addition of DNA nanoparticles in either (C) control conditions or (D) with dynasore treatment significantly reduced down to 55% the number of clathrin-coated particles. (E) At 2 h post-transfection, the mean area of NPs/cell uptaken by differentiated RPE is reduced two-fold in dynasore-treated cells, reflecting that at least half of the nanoparticles are internalized by dynamin-mediated events; remarkably, the mean area of internal NPs is higher in cells transfected by DNA-wrapped NPs compared to pristine NPs. (F) Percentage of NPs/cell in clathrin-coated vesicles is highly similar in all conditions, ranging between 40 and 50%, indicating that a large pool of NPs was not uptaken by RPE cells using this route. Representative images from three independent replicates (three images per replicate and condition) were quantified. Statistical significance was analysed by the non-parametric Mann–Whitney test (* indicates p < 0.05).