Abstract
In this study, a silver doped mesoporous silica nanoparticles-based enzyme-less electrochemical sensor for the determination of hydrogen peroxide (H2O2) released from live cells was constructed for the first time. The presented electrochemical sensor exhibited fast response (2 s) towards the reduction of H2O2 concentration variation at an optimized potential of −0.5 V with high selectivity over biological interferents such as uric acid, ascorbic acid, and glucose. In addition, a wide linear range (4 μM to 10 mM) with a low detection limit (LOD) of 3 μM was obtained. Furthermore, the Ag-mSiO2 nanoparticles/glass carbon electrode (Ag-mSiO2 NPs/GCE) based enzyme-less sensor showed good electrocatalytic performance, as well as good reproducibility, and long-term stability, which provided a successful way to in situ determine H2O2 released from live cells. It may also be promising to monitor the effect of reactive oxygen species (ROS) production in bacteria against oxidants and antibiotics.
Keywords: silver-mesoporous silica nanoparticles, hydrogen peroxide, electrochemical sensor, live cells
1. Introduction
The rapid, specific, and accurate hydrogen peroxide (H2O2) determination is quite essential in bioanalytical fields. H2O2, as the most common representative of reactive oxygen species, which endogenously produced in a cell, is a key player in various pathological processes. High levels of H2O2 results in not only tissue and DNA damage, but also aging, diabetes, cancer, traumatic brain injury, and neurodegenerative disorders [1,2]. Consequently, the determination of H2O2 attracted much interest as its potential as an indicator of oxidative stress-related diseases. Several analytical methods such as spectrophotometry [3], fluorimetry [4], titrimetry [5], chromatography [6], and electrochemistry [7,8] have been applied for highly sensitive H2O2 determination. Amongst the above techniques, electrochemical methods are always optimal choices, due to their high sensitivity, operational simplicity, fast response, low cost, portability, and multi-analyte detection [9,10]. However, most electrode modifiers possessed high potentials, which limited the sensitive and selective performance of H2O2 at ordinary solid electrodes [11]. Thus, the electrode modification is of great importance to minimize the overpotential of redox reaction and improve the rate of electron transfer.
Reportedly, noble metal nanoparticles [12], noble metal nanoparticles on carbon nanostructures [13], and bimetallic nanoparticles [14] have been used for the electrode surfaces modification because of their unique catalytic and electronic properties. The constructed electrochemical sensors performed a highly electrocatalytic activity toward H2O2 reduction or oxidization. Compared with the enzyme-based sensor, enzyme-less sensors are specific to analyte and overcome the limitations from enzyme, such as high cost, susceptible conformation affected by pH, temperature, etc. [15]. Silver nanoparticles (Ag NPs) exhibit good synergetic effect on H2O2 reduction [16,17], which is widely used for enzyme-less electrochemical sensors of H2O2. However, Ag NPs are easy to aggregate in solution before making of electrodes, reducing the sensitivity of the electrochemical sensor. Use of mesoporous silica nanoparticles (mSiO2 NPs) is one of the most effective ways to prevent aggregation [18] because Ag NPs can embed uniformly in the pores of mSiO2 NPs. In addition, the mSiO2 NPs possess unique properties such as large surface area, uniform pore size, ordered mesostructure, and facile surface modification [19,20], which make it a suitable electrically conductive host of Ag NPs to construct electrochemical sensors [21,22]. For example, Khan and Bandyopadhyaya [23] presented an enzyme-less amperometric H2O2 sensor based on Ag NPs impregnated amine functionalized mSiO2 NPs with a wider linearity range (5.3–124.3 mM). Azizi et al. [24] reported a new Ag-doped SBA-16 NPs modified carbon paste electrode, which could achieve detection of H2O2 in two linear ranges (20 μM to 8 mM, and 8 to 20 mM). The biosensor constructed by Ensafi et al. [10] could achieve a lower LOD of 0.45 μM. While the significant success has been made in the detection of traces of H2O2, which is summarized in the new work by Viter and Iatsunskyi [25], biosensors based on Ag-doped MCM-41 mSiO2 NPs for real-time detection of H2O2 released from live cells has not been reported yet, to the best of our knowledge.
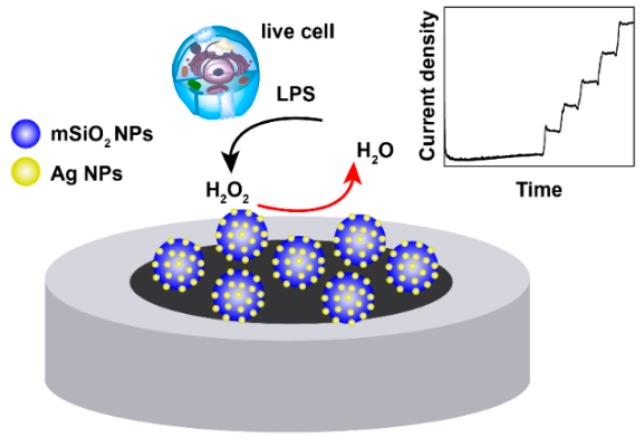
We presented an enzyme-less electrochemical biosensor based on Ag-doped MCM-41 mSiO2 NPs to determine H2O2 released from live cells (Figure 1). Our result showed a high electrocatalytic performance of Ag-mSiO2 nanoparticles/glass carbon electrode (NPs/GCE) towards H2O2 reduction. Additionally, a good linear range from 4 μM to 10 mM with a LOD of 3 μM was obtained. The presented sensor showed high selectivity over biological interferents, such as ascorbic acid, uric acid, and glucose. Moreover, H2O2 released from pheochromocytoma (PC-12) cells could be successfully detected using the present sensor. In addition, as reactive oxygen species (ROS) including H2O2 production in bacteria such as Escherichia coli and Vaginal Lactobacilli can increase the bacteria’s susceptibility to oxidative attack from antibiotic treatment [26,27], our sensor is promising to help understanding the mechanism of H2O2 producing by bacteria against antibiotics in a clinic.
Figure 1.
Schematic of electrode modified with silver-mesoporous silica nanoparticles (Ag-mSiO2 NPs) for H2O2 detection.
2. Materials and Methods
2.1. Materials
Tetraethylorthosilicate (TEOS), hexadecyltrimethylammonium bromide (CTAB), formalin (HCHO, 37%), and silver nitrate (AgNO3) were purchased from Sigma Aldrich. N-(aminoethyl)-amino-propyl trimethoxysilane (TSD) was bought from Aladdin Company. Methanol (CH3OH), disodium hydrogen phosphate (Na2HPO4·12H2O), sodium dihydrogen phosphate (NaH2PO4·2H2O), sodium hydroxide (NaOH), potassium chloride (KCl), potassium ferricyanide (K3Fe(CN)6), ammonium nitrate (NH4NO3), and ethanol (C2H5OH) were purchased from Beijing Chemical Plant. All chemicals were analytical grade. Distilled water (18.2 MΩ cm−1) was used as a solvent.
2.2. Preparation of Ag-mSiO2 Nanoparticles (NPs)
Ag-mSiO2 NPs was prepared following slight modification of the Tian reaction [28]. A quantity of 0.5 mL 2M NaOH was added in 55 mL 7.5 mM CTAB aqueous solution to make solution A; 1 mL TEOS was added to 5 mL methanol to make solution B; 0.25 mL of TSD was added to 5 mL 35 mM AgNO3 solution to make solution C. Under vigorous stirring, solution A was heated to 75 °C and added with 4 mL of solution B dropwise to react for 15 min. Then, solution C and 2 mL of solution B were added dropwise and reacted for 2 h. A total of 2.5 mL formalin solution was finally added and reacted for 1 h. The resulted NPs were removed from CTAB in NH4NO3 ethanol solution (50 mL) at 60 °C for 10 h under stirring. The resulted Ag-mSiO2 NPs were centrifuged, washed with distilled water thrice, and re-suspended into 50 mL deionized water for further use.
2.3. Preparation of H2O2 Biosensors
The surface of a glassy carbon electrode (GCE, 3 mm in diameter) was firstly polished and cleaned to a mirror-like status. Different volumes of Ag-mSiO2 NPs suspension (10, 8, 5, and 3 μL) were dropped respectively on the pretreated electrode surface and dried in the air for 1 h. Then GCE modified with a stable Ag-mSiO2 NPs film (Ag-mSiO2 NPs/GCE) was used for the following electrochemical experiments.
2.4. Determination of H2O2
2.4.1. Detection of Standard H2O2 Solution
H2O2 standard solutions with different concentrations were added to 10 mL of 0.2 M phosphate buffered saline (PBS, pH 6.8, saturated with N2) under stirring, for H2O2 detection. The applied potential (−0.40, −0.45, −0.50, and −0.55 V) was optimized for amperometric analysis of H2O2. The background current was firstly recorded in PBS (pH 6.8) without H2O2 and subtracted for H2O2 calibration plot construction.
2.4.2. Detection of H2O2 Released from Pheochromocytoma cells
The pheochromocytoma (PC-12) cells were grown in 75 cm2 flasks containing RPMI-1640 medium including fetal bovine serum (10%), as well as penicillin and streptomycin (100 μg·mL−1) in 5% CO2 atmosphere at 37 °C. When the PC-12 cells reached a 90% confluence growth, they were centrifuged and responded into 4 mL of PBS (0.2 M pH 6.8). The cell-packed pellet of 1.0–2.0 × 105 cells·cm−2 was obtained for amperometric analysis. After achieving a steady background noise, 60 µg·mL−1 lipopolysaccharide (LPS) was added into the cell mixture to stimulate the release of H2O2 from cells. Then 500 unit·mL−1 catalase was added to decomposing H2O2. As a control group, LPS and catalase were added to PBS solution without cells. An optimized potential of −0.5 V was applied to the Ag-mSiO2 NPs-modified GC electrode for amperometric analysis.
2.5. Characterization
High-resolution transmission electron microscopy (HRTEM, CM200UT, Philips, FEI Co., Hillsboro, OR, USA) was used to examine the morphology and distribution of mSiO2 NPs and Ag-mSiO2 NPs. X-ray diffraction (XRD) pattern was analyzed by an X-ray powder diffractometer (D8 Advance, Bruker, Berlin, Germany). Ultraviolet-visible spectroscopy (UV-vis) absorbance spectra were recorded by UV-vis spectrophotometer (Nanjing Feile Instrument Company, Nanjing, China) with a wavelength range from 300 to 800 nm.
Cyclic voltammetric and amperometric analysis were performed on a CHI660E electrochemical analyzer (Chenhua Co., Shanghai, China) at room temperature. A bare Glassy Carbon (GC) electrode or Ag-mSiO2 NPs modified GC electrode, a Platinum foil electrode and saturated calomel electrode (SCE), were used as the working, the counter and the reference electrode, respectively. Before the detection of H2O2, the solutions were purged by high purity N2 for 30 min to remove oxygen.
3. Results
3.1. Characterization of Ag-mSiO2 NPs
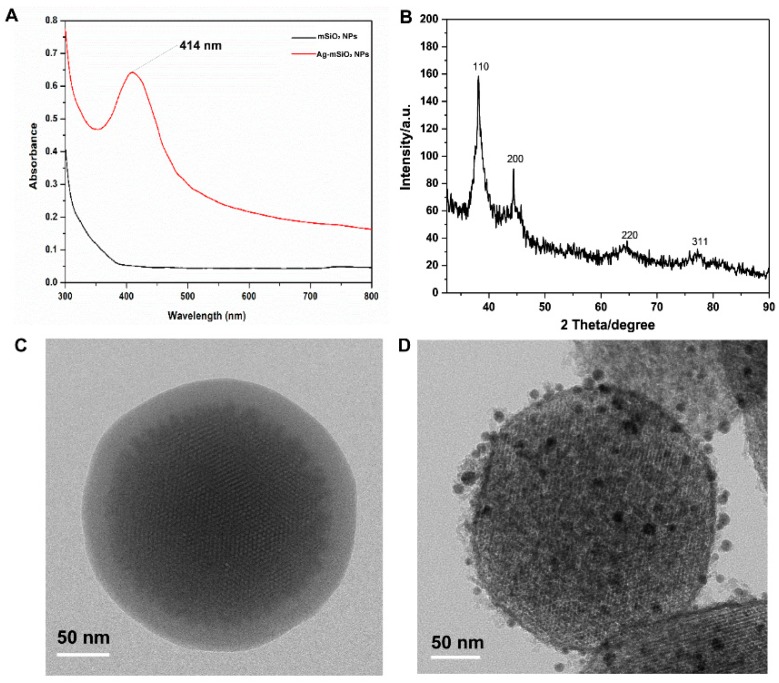
Figure 2A shows the UV-vis absorption spectra of mSiO2 NPs and Ag-mSiO2 NPs. The UV-vis absorbance spectrum of mSiO2 NPs at 414 nm was enhanced after Ag NPs decorated. In the wide-angle XRD pattern of the Ag-mSiO2 NPs (Figure 2B), four well-resolved diffraction peaks at 2θ values in the range of 30°–90° can be indexed to face-centered cubic (fcc) Ag 111, 200, 220, and 311 reflections [29]. The typical morphology and structure of mSiO2 and Ag-mSiO2 NPs was observed clearly through HRTEM. Small Ag NPs were doped in the ordered porous framework of spherical Ag-mSiO2 NPs (Figure 2C,D), which protect themselves from aggregation.
Figure 2.
Characterization of mSiO2 NPs and Ag-mSiO2 NPs. (A) UV-vis-NIR absorption spectrum. (B) Wide-angle XRD patterns. (C) High-resolution transmission electron microscopy (HRTEM) of mSiO2 NPs. (D) HRTEM of Ag-mSiO2 NPs.
3.2. Electrocatalytic Reduction of H2O2
The electrocatalytic activity of the Ag-mSiO2 NPs modified GCE toward the H2O2 reduction was evaluated in PBS (0.2 M, pH 6.8) with a scan rate of 50 mV·s-1. Different volumes of Ag-mSiO2 NPs (3 μL, 5 μL, 8 μL, and 10 μL) modified on the electrode for better cyclic voltammetric (CV) responses were investigated. The modifier of 8 μL on the surface showed the best response, which was applied for the following experiment (Figure S1). The mechanism of H2O2 reduced by Ag-doped mesoporous NPs is as follows [24]:
H2O2 + e− ↔ OHads + OH−
OHads + OH− ↔ OH−
2OH− + 2H+ ↔ 2H2O
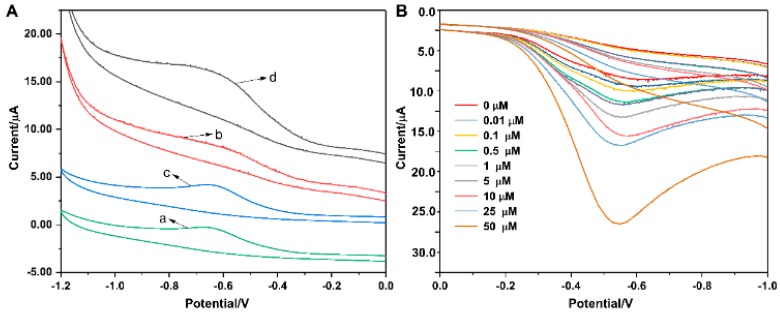
Figure 2A shows the cyclic voltammogram of bare GC electrode (curve a and c) and Ag-mSiO2 modified GC electrode (curve b and d) in PBS (0.2 M, pH 6.8) with the absence or presence of 0.1 mM H2O2. In the absence of H2O2, no obvious cathodic current was observed for bare GCE or Ag-mSiO2 modified GCE (Figure 3Aa,b). However, after the addition of H2O2, the reduction current of Ag-mSiO2 modified GC electrode (Figure 3Ad) greatly increased, while the response signal of the bare GC electrode was very weak (Figure 3Ac). Cyclic voltammogram of Ag-mSiO2 NPs modified GC electrode for H2O2 reduction at various concentrations of H2O2 was shown in Figure 3B. The result revealed that the current responses increased with an increased H2O2 concentration, indicating a good electrocatalytic behavior of Ag-mSiO2/GCE towards H2O2 reduction.
Figure 3.
(A) Cyclic voltammogram of bare glass carbon electrode (GCE) (a,c) and Ag-mSiO2/GCE (b,d) in 0.2 M PBS at pH 6.8 in the absence and the presence of 0.1 mM H2O2, respectively. (B) Current–potential curves of the Ag-mSiO2/GCE for electrocatalytic reduction of H2O2 at the scan rate of 50 mV s−1 in 0.2 M PBS solution with different concentrations of H2O2, respectively.
3.3. Amperometric Determination of H2O2
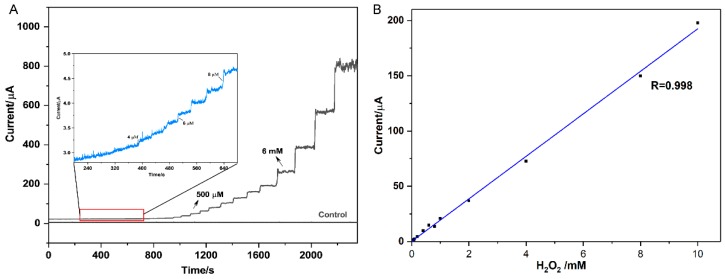
The amperometric analysis was used to evaluate the electrocatalytic activity of Ag-mSiO2/GC electrode for different concentrations of H2O2. First of all, the effect of applied potential on amperometric current-time (i−t) curve was studied. Several applied potentials of −0.40, −0.45, −0.50, and −0.55 V was employed to investigate the according amperometric response of Ag-mSiO2/GC electrode in PBS (0.2 M, pH 6.8) with the successive addition of 1.0 mM H2O2. The amperometric response reached a maximum value at a potential of −0.50 V (Figure S2), which was selected for the following amperometric analysis. Figure 4A showed the amperometric i–t curves of Ag-mSiO2 NPs/GC electrode with the successive additions of H2O2 into deoxygenated PBS (0.2 M, pH 6.8) under continuous stirring. Before adding H2O2, the amperometric current of Ag-mSiO2 NPs/GC electrode was recorded in PBS solution for 200 s to obtain a steady blank control. The Ag-mSiO2 NPs/GCE-based sensor could reach a steady-state current within 2 s after a small amount addition of H2O2, which indicated its fast response for H2O2 reduction. Additionally, a wide linear range from 4 μM to 10 mM was obtained. The LOD is calculated to be 3 μM (S/N = 3) (Figure 4B). The present H2O2 biosensor revealed a wide linear range, low LOD, and fast response.
Figure 4.
(A) Current–time (i−t) data and (B) calibration plot for H2O2 for Ag-mSiO2/GC electrode. Inset in (A) shows current–time response for 4, 6, and 8 μmoL H2O2 (arrows in black indicate point of aliquot additions).
3.4. Interferences Study
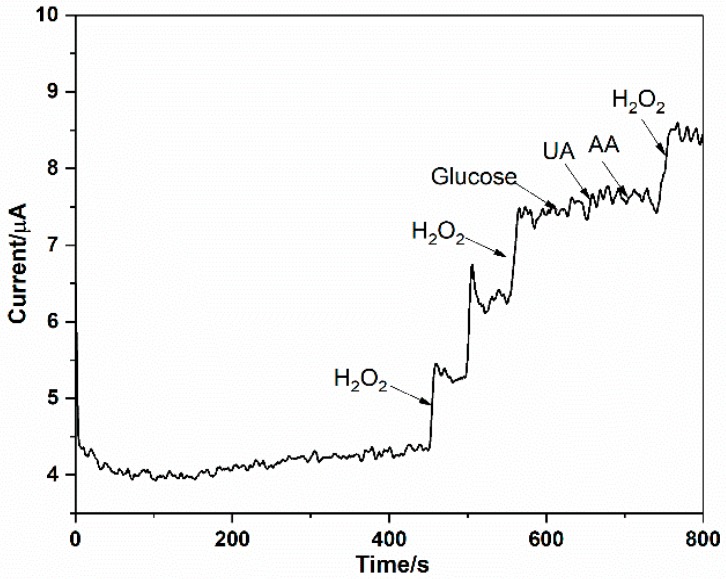
Glucose, ascorbic acid (AA), and uric acid (UA) are three commonly interferents in a physiological system. The anti-interference effect of the Ag-mSiO2 NPs /GCE based sensor toward glucose, AA, and UA was investigated. The amperometric responses of the present sensor with sequential addition of 0.05 mM H2O2, 1 mM glucose, 0.15 mM AA, and 0.5 mM UA are shown in Figure 5. It can be seen that a large amperometric response was achieved with a low concentration of 0.05 mM H2O2, while negligible current was observed with high concentrations of the other interfering species such as glucose, AA or UA. This showed the present sensor was selective to H2O2.
Figure 5.
Current-time response curve at Ag-mSiO2/GCE for successive injection of H2O2, glucose, UA, AA in 0.2 M PBS at −0.5 V.
3.5. Reproducibility and Stability
The reproducibility of Ag-mSiO2 NPs modified single electrode was tested for five successive measurements with the relative standard derivation (RSD) in about 2.6%. Moreover, the RSD of current responses of five electrodes was investigated and found to be less than 5%, suggesting the high reproducibility and good precision of our present sensor. Furthermore, the current responses of the sensor decreased to 90.2% of its original value after being stored in the refrigerator at 4 °C for 20 days. The above results indicated the reliability and long-term stability of our fabricated H2O2 sensor. In addition, we compared the hydrogen peroxided sensing by AgNP impregnated silica NPs in Table 1. H2O2 sensor reported here has a wide linear range and low detection limit and can detect the H2O2 released from living cells.
Table 1.
Comparison of the present work with that reported in literature for hydrogen peroxide sensing by Ag NP impregnated silica NPs.
| Types of Electrode | Applied Potential (V) | Linear Range (µM) | Detection Limit (µM) | Samples | Reference |
|---|---|---|---|---|---|
| AgNP-NH2-SBA-15-GCE | −0.4 vs. SCE | 0.49–5.3 5.3–124.5 |
N.A. | PBS buffer | [19] |
| Ag/SBA-16/CPE | −0.45 vs. Ag|AgCl | 20–8000 8000–20,000 |
2.95 | Hair dying cream | [20] |
| Ag@SiO2 YSNs | −0.5 vs. Ag|AgCl | 100–15,000 | 3.5 | PBS buffer | [18] |
| Ag NPs/porous silicon | −0.45 vs. Ag|AgCl | 1.65–500 | 0.45 | Hair color oxidant | [10] |
| Nanoporous silver/Ni wire | −0.35 vs. SCE | 10.0–8000 | 4.80 | PBS buffer | [30] |
| Ag-mSiO2 NPs/GCE | −0.5 vs. SCE | 4–10,000 | 3 | Live cell | This work |
3.6. Determination of H2O2 Released from Pheochromocytoma Cells
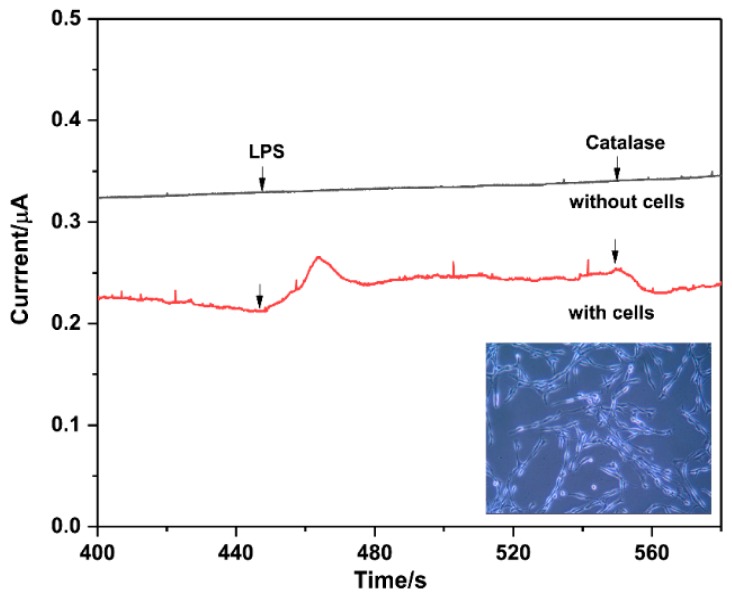
Moreover, we performed a study of the real-time determination of H2O2 released from live cells. For the experiment, about 2 × 105 pheochromocytoma (PC-12) cells in 4 mL PBS (pH = 6.8) with 100 mM glucose were used. LPS (60 μg mL−1) was employed to stimulate PC-12 cells to generate H2O2. After treating with LPS, an obvious current change occurred in solution with cells, while no signal could be observed in that without cells. We also can see from Figure 6 that the current decreased rapidly with an injection of 500 unit·mL−1 catalase. As catalase is a selective scavenger of H2O2, it can be concluded that the increased current in the curve “with cells” was assigned to the reduction of H2O2.
Figure 6.
Amperometric responses of the Ag-mSiO2/GCE in 0.2 M PBS (pH 6.8) with the addition of LPS and catalase in the absence (upper curve) and presence (lower curve) of PC 12 cells.
4. Conclusions
In summary, a highly sensitive and selective enzyme-less sensor of H2O2 was successfully constructed with Ag-mSiO2 NPs/GCE, enabling good electrocatalytic performance toward H2O2 reduction. The modified electrode exhibited a fast response to H2O2 concentration variation at an optimized working potential of −0.5 V. A wide linear range from 4 μM to 10 mM with a low LOD of 3 μM was obtained. In addition, the presented sensor showed high selectivity over commonly interfering substances, such as uric acid (UA), ascorbic acid (AA), and glucose. Furthermore, the Ag-mSiO2 NPs/GCE based enzyme-less sensor exhibits good reproducibility and long-term stability. Accordingly, the constructed biosensor could be successfully used for the determination of H2O2 released from live cells. As ROS or H2O2 has been associated with cell cycle arrest, apoptosis, migration, and inflammation, or the antibacterial activity of certain bacteria such as Lactobacilli, our sensor has potential to help understand the role of H2O2 that plays in the biological and pathological systems. However, for monitoring the ROS or H2O2 from real samples, the stability of the constructed sensor still needs to be improved with employing more advanced nanomaterials because there is a lot of interferent in real clinical samples.
Acknowledgments
We acknowledge technical support from Jun Zhou for Wide-angle XRD patterns analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-666X/10/4/268/s1, Figure S1: current–potential curves of the Ag-mSiO2 NPs/GCE for electrocatalytic reduction of H2O2 at the scan rate of 50 mV s−1 in 0.2 M PBS solution with different modifiers of Ag-mSiO2; Figure S2: The amperometric response of Ag-mSiO2 nanoparticles/glass carbon electrode (NPs/GCE) (with applied potentials of −0.40, −0.45, −0.50, and −0.55 V) in PBS solution (0.2 M, pH 6.8) upon successive additions of 1.0 mM H2O2.
Author Contributions
Investigation, N.N. and X.S.; methodology, L.C.; validation, G.Y., Y.A.; writing—original draft, D.Y.; writing—review and editing, J.Z., H.Z.
Funding
This research is funded by National Natural Science Foundation of China (grant number 81773684), Natural Science Foundation of Zhejiang Province (grant number LY17H260003), Guangdong Natural Science Funds for Distinguished Young Scholars (2018B030306033), Pearl River S&T Nova Program of Guangzhou (201806010060), Ningbo Natural Science Foundation (grant number 2017A610226), Science Program of Ningbo University (grant number: XYL17021) and K.C. Wong Magna Fund in Ningbo University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Luo Y., Liu H., Rui Q., Tian Y. Detection of Extracellular H2O2 Released from Human Liver Cancer Cells Based on TiO2 Nanoneedles with Enhanced Electron Transfer of Cytochrome c. Anal. Chem. 2009;81:3035–3041. doi: 10.1021/ac802721x. [DOI] [PubMed] [Google Scholar]
- 2.Weinstain R., Savariar E.N., Felsen C.N., Tsien R.Y. In vivo targeting of hydrogen peroxide by activatable cell-penetrating peptides. J. Am. Chem. Soc. 2014;136:874–877. doi: 10.1021/ja411547j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Min Z., Huang X., Liu L., Shen H. Spectrophotometric determination of hydrogen peroxide by using the cleavage of Eriochrome black T in the presence of peroxidase. Talanta. 1997;44:1407–1412. doi: 10.1016/S0039-9140(97)00012-X. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport R., Hanukoglu I., Sklan D. A fluorimetric assay for hydrogen peroxide, suitable for NAD(P)H-dependent superoxide generating redox systems. Anal. Biochem. 1994;218:309–313. doi: 10.1006/abio.1994.1183. [DOI] [PubMed] [Google Scholar]
- 5.Klassen N.V., Marchington D., McGowan H.C.E. H2O2 Determination by the I3- Method and by KMnO4 Titration. Anal. Chem. 1994;66:2921–2925. doi: 10.1021/ac00090a020. [DOI] [Google Scholar]
- 6.Effkemann S., Pinkernell U., Karst U. Peroxide analysis in laundry detergents using liquid chromatography. Anal. Chim. Acta. 1998;363:97–103. doi: 10.1016/S0003-2670(98)00040-3. [DOI] [Google Scholar]
- 7.Yu G., Wu W., Pan X., Zhao Q., Wei X., Lu Q. High sensitive and selective sensing of hydrogen peroxide released from pheochromocytoma cells based on Pt-Au bimetallic nanoparticles electrodeposited on reduced graphene sheets. Sensors. 2015;15:2709–2722. doi: 10.3390/s150202709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T., Zhu H., Zhuo J., Zhu Z., Papakonstantinou P., Lubarsky G., Lin J., Li M. Biosensor based on ultrasmall MoS2 nanoparticles for electrochemical detection of H2O2 released by cells at the nanomolar level. Anal. Chem. 2013;85:10289–10295. doi: 10.1021/ac402114c. [DOI] [PubMed] [Google Scholar]
- 9.Miao P., Wang B., Yin J., Chen X., Tang Y. Electrochemical tracking hydrogen peroxide secretion in live cells based on autocatalytic oxidation reaction of silver nanoparticles. Electrochem. Commun. 2015;53:37–40. doi: 10.1016/j.elecom.2015.02.007. [DOI] [Google Scholar]
- 10.Ensafi A.A., Rezaloo F., Rezaei B. Electrochemical sensor based on porous silicon/silver nanocomposite for the determination of hydrogen peroxide. Sens. Actuat. B—Chem. 2016;231:239–244. doi: 10.1016/j.snb.2016.03.018. [DOI] [Google Scholar]
- 11.Katsuki H., Komarneni S. Synthesis of Na-A and/or Na-X zeolite/porous carboncomposites from carbonized rice husk. J. Solid State Chem. 2009;182:1749–1753. doi: 10.1016/j.jssc.2009.04.022. [DOI] [Google Scholar]
- 12.Pingarrón J.M., Yáñez-Sedeño P., González-Cortés A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta. 2008;53:5848–5866. doi: 10.1016/j.electacta.2008.03.005. [DOI] [Google Scholar]
- 13.Ju J., Chen W. In situ growth of surfactant-free gold nanoparticles on nitrogen-doped graphene quantum dots for electrochemical detection of hydrogen peroxide in biological environments. Anal. Chem. 2015;87:1903–1910. doi: 10.1021/ac5041555. [DOI] [PubMed] [Google Scholar]
- 14.Yi L., Wei W., Zhao C., Yang C., Tian L., Liu J., Wang X. Electrochemical oxidation of sodium borohydride on carbon supported Pt-Zn nanoparticle bimetallic catalyst and its implications to direct borohydride-hydrogen peroxide fuel cell. Electrochim. Acta. 2015;158:209–218. doi: 10.1016/j.electacta.2015.01.111. [DOI] [Google Scholar]
- 15.Huang F., Xue L., Zhang H., Guo R., Li Y., Liao M., Wang M., Lin J. An enzyme-free biosensor for sensitive detection of Salmonella using curcumin as signal reporter and click chemistry for signal amplification. Theranostics. 2018;8:6263–6273. doi: 10.7150/thno.29025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maduraiveeran G., Kundu M., Sasidharan M. Electrochemical detection of hydrogen peroxide based on silver nanoparticles via amplified electron transfer process. J. Mater. Sci. 2018;53:8328–8338. doi: 10.1007/s10853-018-2141-7. [DOI] [Google Scholar]
- 17.Baghayeri M., Veisi H., Farhadi S., Beitollahi H., Maleki B. Ag nanoparticles decorated Fe3O4/chitosan nanocomposite: Synthesis, characterization and application toward electrochemical sensing of hydrogen peroxide. J. Iran. Chem. Soc. 2018;15:1015–1022. doi: 10.1007/s13738-018-1298-y. [DOI] [Google Scholar]
- 18.Kresge C.T., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359:710–712. doi: 10.1038/359710a0. [DOI] [Google Scholar]
- 19.Tian C., Li J., Ma C., Wang P., Sun X., Fang J. An ordered mesoporous Ag superstructure synthesized via a template strategy for surface-enhanced Raman spectroscopy. Nanoscale. 2015;7:12318–12324. doi: 10.1039/C5NR03759J. [DOI] [PubMed] [Google Scholar]
- 20.Park J.H., Park J.K., Shin H.Y. The preparation of Ag/mesoporous silica by direct silver reduction and Ag/functionalized mesoporous silica by in situ formation of adsorbed silver. Mater. Lett. 2007;61:156–159. doi: 10.1016/j.matlet.2006.04.118. [DOI] [Google Scholar]
- 21.Han J., Fang P., Jiang W., Li L., Guo R. Ag-nanoparticle-loaded mesoporous silica: Spontaneous formation of Ag nanoparticles and mesoporous silica SBA-15 by a one-pot strategy and their catalytic applications. Langmuir. 2012;28:4768–4775. doi: 10.1021/la204503b. [DOI] [PubMed] [Google Scholar]
- 22.Liu C., Li J., Wang J., Qi J., Fan W., Shen J., Sun X., Han W., Wang L. Synthesis of Ag@SiO2 yolk-shell nanoparticles for hydrogen peroxide detection. RSC Adv. 2015;5:17372–17378. doi: 10.1039/C4RA16061D. [DOI] [Google Scholar]
- 23.Khan A.Y., Bandyopadhyaya R. Silver nanoparticle impregnated mesoporous silica as a non-enzymatic amperometric sensor for an aqueous solution of hydrogen peroxide. Electroanal. Chem. 2014;727:184–190. doi: 10.1016/j.jelechem.2014.05.027. [DOI] [Google Scholar]
- 24.Azizi S.N., Ghasemi S., Maybodi A.S., Azad M.R. A new modified electrode based on Ag-doped mesoporous SBA-16 nanoparticles as non-enzymatic sensor for hydrogen peroxide. Sens. Actuat. B-Chem. 2015;216:271–278. doi: 10.1016/j.snb.2015.03.078. [DOI] [Google Scholar]
- 25.Viter R., Iatsunskyi I. In: Nanomaterials Design for Sensing Applications. Zenkina O.V., editor. Elsevier; Amsterdam, The Netherlands: 2019. pp. 41–91. Chapter 2: Metal Oxide Nanostructures in Sensing. [DOI] [Google Scholar]
- 26.Brynildsen M.P., Winkler J.A., Spina C.S., MacDonald I.C., Collins J.J. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nat. Biotech. 2013;31:160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sgibnev A., Kremleva E. Influence of Hydrogen Peroxide, Lactic Acid, and Surfactants from Vaginal Lactobacilli on the Antibiotic Sensitivity of Opportunistic Bacteria. Probiot. Antimicrob. Proteins. 2017;9:131. doi: 10.1007/s12602-016-9238-6. [DOI] [PubMed] [Google Scholar]
- 28.Tian Y., Qi J., Zhang W., Cai Q., Jiang X. Facile, one-pot synthesis, and antibacterial activity of mesoporous silica nanoparticles decorated with well-dispersed silver nanoparticles. ACS Appl. Mater. Interfaces. 2014;6:12038–12045. doi: 10.1021/am5026424. [DOI] [PubMed] [Google Scholar]
- 29.Kumari M., Jacob J., Philip D. Green synthesis and applications of Au–Ag bimetallic nanoparticles. Spectrochim. Acta A. 2015;137:185–192. doi: 10.1016/j.saa.2014.08.079. [DOI] [PubMed] [Google Scholar]
- 30.Zou X., He Y., Sun P., Zhao J., Cui G. A novel dealloying strategy for fabricating nanoporous silver as an electrocatalyst for hydrogen peroxide detection. Appl. Surf. Sci. 2018;447:542–547. doi: 10.1016/j.apsusc.2018.04.018. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.