Fig. 2.
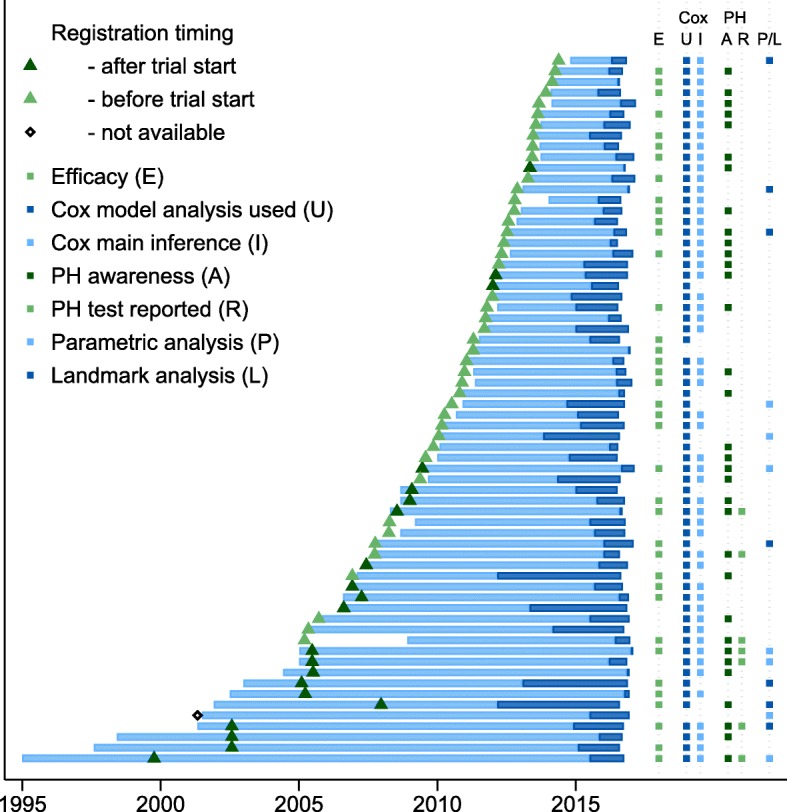
Summary presentation of the findings of the review. Trial duration (years), between nominated start date and completion date, is indicated by the lighter shaded horizontal bars. Duration of time between completion and publication data is indicated by the darker shaded horizontal bars. Time of trial registration is shown by the triangles with lighter and darker shading indicating registration before and after nominated trial start date. Columns on the right side represent the determinations of trial characteristics for this review, including a trial reporting efficacy (E) of the primary outcome, the Cox PH model usage (U) in the report and presentation of the hazard ratio as the main inferential (I) finding. For trials using Cox analysis, the determinations of the awareness (A) and reporting (R) of the proportional hazards assumption for each trial is presented. Planned or presented usage of alternative regression models to the Cox PH model such as parametric or landmark (P/L) analysis is shown in the final column
