Abstract
Dendrites form an essential component of the neuronal circuit have been largely overlooked in regenerative research. Nevertheless, subtle changes in the dendritic arbors of neurons are one of the first stages of various neurodegenerative diseases, leading to dysfunctional neuronal networks and ultimately cellular death. Maintaining dendrites is therefore considered an essential neuroprotective strategy. This mini-review aims to discuss an intriguing hypothesis, which postulates that dendritic shrinkage is an important stimulant to boost axonal regeneration, and thus that preserving dendrites might not be the ideal therapeutic method to regain a full functional network upon central nervous system damage. Indeed, our study in zebrafish, a versatile animal model with robust regenerative capacity recently unraveled that dendritic retraction is evoked prior to axonal regrowth after optic nerve injury. Strikingly, inhibiting dendritic pruning upon damage perturbed axonal regeneration. This constraining effect of dendrites on axonal regrowth has sporadically been proposed in literature, as summarized in this short narrative. In addition, the review discusses a plausible underlying mechanism for the observed antagonistic axon-dendrite interplay, which is based on energy restriction inside neurons. Axonal injury indeed leads to a high local energy demand in which efficient axonal energy supply is fundamental to ensure regrowth. At the same time, axonal lesion is known to induce mitochondrial depolarization, causing energy depletion in the axonal compartment of damaged neurons. Mitochondria, however, become mostly stationary after development, which has been proposed as a potential underlying reason for the low regenerative capacity of adult mammals. Per contra, upon reduced neuronal activity, mitochondrial mobility enhances. In this view, dendritic shrinkage after axonal injury in zebrafish could result in less synaptic input and hence, a release of mitochondria within the soma-dendrite compartment that then translocate to the axonal growth cone to stimulate axonal regeneration. If this hypothesis proofs to be correct, i.e. dendritic remodeling serving as fuel for axonal regeneration, we envision a major shift in the research focus within the neuroregenerative field and in the potential uncovering of various novel therapeutic targets.
Keywords: axonal regeneration, dendritic remodeling, retina, central nervous system, zebrafish, mitochondrial transport, mitochondrial dynamics, energy supply
Critical for correct neural circuit functioning is the transfer of electrical signals from one neuron to another via synaptic transmission. It is therefore not surprising that early-stage pathology in various neurodegenerative diseases is not characterized by a massive neuronal cell death but defined by subtle changes in neurites, specifically resulting in synapto-dendritic degeneration and dendrite pruning. Also glaucomatous optic neuropathies are characterized by early retinal synapse loss, retinal ganglion cell (RGC) dendritic shrinkage, and a reduced dendritic complexity, which likely precede irreversible structural damage to the optic nerve and RGC death. Maintaining dendrites is therefore proposed as an important neuroprotective strategy for preserving functional RGCs. From a clinical point of view, however, this seems unrealistic as RGC dendrite abnormalities presumably manifest even before diagnosis is possible. Neuroprotection will thus fall short for restoring a functional neuronal circuitry and must therefore be complemented by dendritic regeneration. If and how neurons are capable to regrow dendrites after damage remains largely elusive, as research concerning vertebrate dendrite regeneration is still in its infancy, with only two papers in this field. In a first study, dendrite regeneration was induced after stereotaxic prick-injury in the adult cerebral cortex of mice, by applying heparin-binding growth-associated molecule, which modulates the glial scar (Paveliev et al., 2016). A second study reported that restoring mechanistic target of rapamycin (mTOR) activity using intravitreal insulin injection resulted in a prominent dendrite-regenerating effect after axotomy-induced dendrite retraction in mouse RGCs (Agostinone et al., 2018). Unfortunately, both studies only focused on improving dendrite regeneration after injury, leaving the effect on axonal regeneration unmonitored. In this review the role of dendrite remodeling in axonal regeneration and the possible link with mitochondrial dynamics is discussed, based on own published data (Beckers et al., 2018) and on a broad PubMed literature search of articles published in the period 2010–2019 on dendrite remodeling and on the role of mitochondria in axonal regeneration.
Successful dendrite regeneration has also been occasionally reported in different invertebrate species. In Caenorhabditis elegans, in vivo laser-induced dendritomy of sensory PVD neurons triggered the severed primary dendrite to regrow both the distal and proximal stump and reconnect via fusion. This capacity to regrow and auto-fuse dendrites after injury declined with age but could be restored in e.g. mutants for daf-2, an insulin-like growth factor receptor (Kravtsov et al., 2017). In Drosophila, dendritic arborization sensory neurons also displayed robust dendrite regeneration upon laser injury in vivo, in which certain neuron classes regrew until the normal dendritic coverage was reached, while others stopped when the number of initial branch points was restored (Stone et al., 2014). C. elegans and Drosophila thus have a robust ability to regenerate axons, as well as their dendrites, enabling functional nervous system repair after injury.
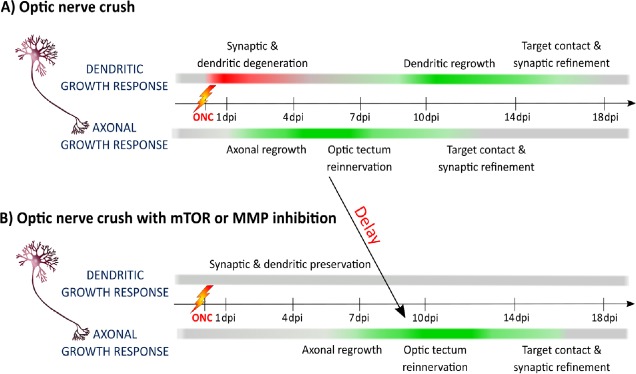
Although some invertebrate and vertebrate neurons thus seem to have an intrinsic dendrite regeneration potential, a possible interplay between axonal and dendritic regeneration has not been studied. To investigate such an interaction, we used a vertebrate animal model that is capable of spontaneous central nervous system (CNS) axonal regeneration. Adult zebrafish are indeed well known to regenerate their RGC axons after optic nerve crush (ONC), eventually leading to full restoration of vision. At first, we investigated whether optic nerve injury also triggers retinal dendrite retraction, similar as in mammals and if they can subsequently repair these neurites. Immediate synaptic degeneration and dendrite shrinkage was clearly observed in zebrafish subjected to ONC, indicated as a negative dendritic growth response in Figure 1A (Beckers et al., 2018). Remarkably, axonal regeneration was only initiated afterwards, i.e. when extensive synaptic and dendritic deterioration already occurred (Figure 1A). The regrowing axons eventually reinnervated their target neurons in the optic tectum, which then triggered the retinal dendrites to regrow and reconnect, leading to a restored functional neuronal circuit and repair of vision (Figure 1A) (Beckers et al., 2018). Together these findings indicate that spontaneously regenerating vertebrate neurons also retract their dendrites after axonal injury, similar to the mammalian situation, but that they have the ability to spontaneously restore both axons and dendrites, a feature clearly missing in mammals. This remarkable dendrite-regenerative capacity in adult zebrafish neurons has already been described in other retinal cells, namely the bipolar cells. After intravitreal injection of ouabain, a cytotoxin known to destroy the inner retina, bipolar cells were shown able to repair synaptic connections and dendrite morphology (McGinn et al., 2018). Zebrafish retinal neurons thus have the intrinsic ability to regenerate both axons and dendrites.
Figure 1.
Schematic representation of the RGC axonal and dendritic growth response in relation to time after ONC in untreated or mTOR/MMP inhibited adult zebrafish.
(A) Immediately after injury, retinal synapses degenerate and RGC dendrites retract, depicted as a negative growth response, shown in red. Directly after dendrite shrinkage, RGC axons start to regrow, inducing a positive growth response, shown in green, eventually resulting in optic tectum reinnervation and target contact initiation. This, in turn, triggers the dendrites to shift their negative growth response to a positive one and to establish new synaptic contacts, finally leading to restoration of vision. (B) Retinal mTOR or MMP inhibition prevents retinal synapse degradation and dendrite collapsing after ONC, shown in a grey color for an unchanged growth response. This dendritic preservation consecutively delays the initiation of axonal regrowth, resulting in a retarded optic tectum reinnervation. Here the effect on target contact initiation was not studied. ONC: Optic nerve crush; RGC: retinal ganglion cell; dpi: day(s) post-injury; mTOR: mechanistic target of rapamycin; MMP: matrix metalloproteinases.
The well-ordered and timed organization of dendritic remodeling and axonal regrowth during CNS regeneration in zebrafish suggests an antagonistic and hence interdependent axon-dendrite interplay. As such, we investigated whether inhibition of dendritic shrinkage affects axonal regeneration, first by focusing on mTOR. This kinase, important for energy and nutrient monitoring, is a key player in zebrafish and mammalian RGC axonal regeneration and also involved in dendrite development and regeneration (Beckers et al., 2018). mTOR is transiently activated in zebrafish RGCs subjected to ONC (Beckers et al., 2018). Strikingly, mTOR inhibition via intravitreal injections of rapamycin resulted in the preservation of synapses and dendrites early after ONC, which subsequently perturbed optic tectum reinnervation (Figure 1B). Delayed rapamycin treatment, i.e. at a time that synapses and dendrites were already degraded, did not negatively influence axonal regeneration, further supporting the finding that dendritic shrinkage is favorable for axonal regeneration. Finally, inhibition of broad-spectrum matrix metalloproteinases, also important for axonal regeneration and dendritic remodeling, revealed a similar effect, namely that synapto-dendritic deterioration and axonal regrowth were consecutively inhibited/delayed (Figure 1B) (Beckers et al., 2018). All in all, these data clearly suggest an antagonistic reciprocity between axonal regeneration and dendritic remodeling during CNS repair.
Our findings in which dendritogenesis precedes axogenesis, is reminiscent of what happens during RGC development, where there is indeed a distinct temporal time window and order in which neurite outgrowth occurs. Newborn RGCs first project axons towards the brain and upon target contact, dendrites emerge in the retina to connect with bipolar and horizontal cells. Moreover, it has been proposed that a contact-mediated or membrane-associated signal from amacrine cells provokes an irreversible switch between an axonal and a dendritic growth mode. The obtained data could thus indicate that dendritic shrinkage in zebrafish RGCs is helpful to reverse the dendritic growth mode to an axonal one and repeat the developmental neurite outgrowth order, in which axonal regeneration is prioritized over dendrite regrowth. A constraining effect of dendrites on axonal regeneration is also reported in C. elegans. Here, axonal regeneration of ASJ neurons was enhanced when axotomy was performed simultaneously with dendritomy, as compared to axonal injury only (Chung et al., 2016). In this view, it seems plausible that also in mammals, dendritic shrinkage after axonal damage is the proper preparatory step for axonal regeneration, which is thereafter unfortunately nullified due to the lack of proper intrinsic and extrinsic growth factors and a strong inhibitory environment. This idea is reinforced by the recent finding that intravitreal administrating ciliary neurotrophic factor (CNTF), which promotes axonal regeneration, in mice after axotomy, was found to be accompanied with a more severe reduction in dendritic arbor length/complexity compared to axotomy alone, again hinting towards the hypothesis that dendritic shrinkage can boost axonal regrowth (Drummond et al., 2014).
One of the mechanisms underlying the segregation of dendritic and axonal growth during development and regeneration, might be a neuronal energy restriction/trade-off, in which a neuron is not capable of (re)growing axons and producing/maintaining functional dendrites simultaneously. Indeed, axonal generation during development requires a massive amount of energy in the form of adenosine triphosphate (ATP), mainly produced by mitochondria, which accumulate in active growth cones. These growth cones heavily rely on ATP-mediated actin polymerization, providing the pushing force for growth cone protrusion. After axonal injury, mitochondria are however depolarized, which results in dysfunction and less ATP production at the axonal stump. The ideal method to reverse the injury-induced energy deficit would be a translocation of healthy mitochondria towards the injury site, thereby enabling growth cone formation and axon outgrowth initiation.
Unfortunately, while mitochondria are very motile during development, most of them become stationary in mature mammalian CNS neurons in regions with high metabolic needs, such as synapses. This reduction in mitochondrial motility has been put forward as an important limiting factor for CNS axonal regeneration in adult mammals. In fact, it has been reported that animals showing robust regeneration increase their axonal mitochondrial transport rate after injury. In C. elegans, 70% of the axons of γ-aminobutyric acid motor neurons regenerate after laser axotomy in vivo, while 30% fail to form a functional growth cone. The growing axons were characterized by an increased mitochondrial density compared to control or injured non-regenerating axons, and this was found to be due to elevated mitochondrial transport and not enhanced mitochondrial biogenesis/fission or reduced mitophagy. Notably, experimentally in- or decreasing mitochondrial density in these injured axons did not change the percentage of successful growth cone formation but had the expected positive, respectively, negative effect on neurite lengthening in the axons that did regenerate (Han et al., 2016). Another study reported a positive impact of mitochondrial motility on axonal outgrowth in spontaneously regenerating zebrafish larvae. After spinal cord laser axotomy, an increased number of mobile mitochondria was observed in the axons that regenerated over long distance as compared to the short ones, and stimulating axonal outgrowth using dibutyryl cyclic adenosine monophosphate enhanced mitochondrial motility (Xu et al., 2017). Also in the peripheral nervous system (PNS), which is capable to regenerate axons, even in mammalian models, it is reported that mitochondrial transport increases following axonal injury. Indeed, after in vivo axonal transection of the peripheral branch of dorsal root ganglia (DRG) and intercostal nerves of mice, axonal mitochondrial mobility, analyzed in the corresponding explants in vitro, was found to be elevated (Mar et al., 2014). In another study, Zhou et al. (2016) increased mitochondrial transport via deletion of the axonal mitochondria-anchoring protein syntaphilin and revealed an accelerated axonal regeneration after sciatic nerve crush in mice, again providing evidence that mitochondrial transport underlies the axonal regenerative outcome in the mammalian PNS.
In contrast to the PNS, the mammalian CNS does not regenerate spontaneously, but over the years different methods have been developed to experimentally stimulate axonal regeneration. For two of these axonal-inducing paradigms, increased mitochondrial transport was recently identified as an underlying factor for the beneficial effect. Indeed, a regenerative response of the central branch of mouse DRG neurons after spinal cord injury is absent under normal physiological conditions, but can be evoked following a peripheral branch lesion in the form of sciatic nerve injury. This “peripheral lesion condition” boosts global axonal transport including that of mitochondria, both in the peripheral and the central branch, measured using DRG explants. Notably, only severing the central branch of DRG neurons did not increase mitochondrial transport, nor did it lead to axonal regrowth (Mar et al., 2014). Secondly, mice with a co-deletion of phosphate and tensin homolog (PTEN) and suppressor of cytokine signaling 3 (SOCS3), known to robustly enhance RGC axonal regrowth, show an increased expression of armadillo repeat containing X‐linked 1 (Armcx1), a mitochondrial protein that interacts with the transport machinery via mitochondrial Rho GTPase 1 (Cartoni et al., 2017). Subsequent overexpression and knockdown experiments indicated that Armcx1 is crucial for the positive axonal regeneration effect after ONC and does so by releasing stationary mitochondria and thus promoting mitochondrial mobility. Similar results were found using cultured E18 mouse cortical neurons, indicating that the positive effect of Armcx1 on neurite outgrowth and mitochondrial motility was not only RGC-specific. Overall, these data in the mammalian CNS and spontaneously regenerating nervous systems support a crucial role of mitochondrial transport in axonal repair.
Notably, mitochondrial motility is rather low in mammalian adult firing neurons, but enhances upon a reduced synaptic/neuronal activity. Indeed, various studies have highlighted that mitochondrial motility is blocked by action potentials, possibly due to the influx of calcium ions (Cai and Sheng, 2009). These calcium ions bind to Miro within the mitochondrial motor/adaptor complex, provoking a conformational change that disrupts the connection between this complex and the microtubule, with stationary mitochondria as a result. In this view, the observed synapse loss and dendritic shrinkage after axonal injury in zebrafish could result in a reduced synaptic input and hence, a release of mitochondria within the dendrites that then translocate to the axonal growth cone in order to boost axonal regeneration (Figure 2). If this hypothesis proofs to be correct, researchers need to move away from the idea that maintaining and protecting dendrites after axonal damage is the best therapeutic approach, as this could prevent intra-neuronal mitochondrial channeling and hence counteract axonal repair. On the other hand, we cannot exclude that other processes, including mitochondrial fission/fusion, biogenesis or mitophagy might also contribute to the distribution of energy resources in a cell. Anyhow, more research is clearly needed to provide causal evidence for the observed antagonistic axon-dendrite interplay and a role for neuronal energy translocation during CNS regeneration. Overall, recent work does underline the importance of analyzing dendritic responses after axonal injury and the effect of mitochondrial dynamics, which could be a powerful target-discovery platform for neural circuit repair.
Figure 2.
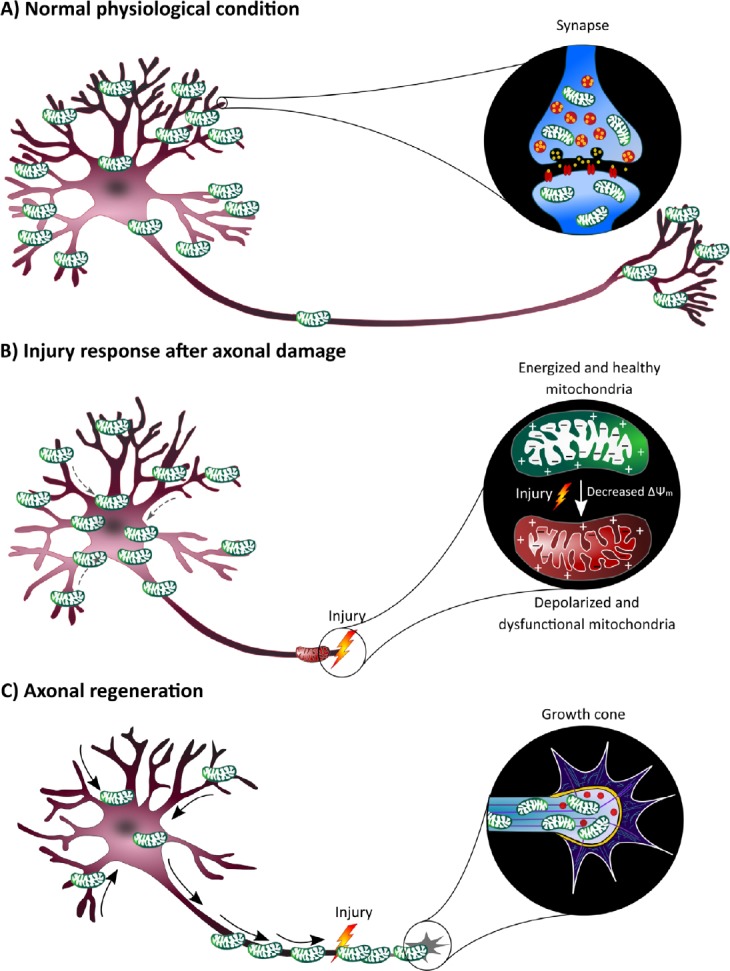
Schematic representation of the hypothesis that dendritic shrinkage after optic nerve crush is accompanied by the mobilization of mitochondria, which could be the driving force for axonal regeneration.
(A) In normal physiological conditions, most mitochondria in neurons are stationary and accumulate at sites with a high energy need, especially in synapses, where they play an essential role in synapse maintenance and neurotransmission. (B) Axonal injury induces a drop in mitochondrial membrane potential (ΔΨm) in the nearby mitochondria, resulting in mitochondrial depolarization. These dysfunctional mitochondria are not able to produce the necessary energy for growth cone formation. Within our literature-based energy trade-off hypothesis, dendrites then start to retract after axonal injury, which could go hand in hand with mitochondrial reshuffling towards the cell soma, as reduced synaptic input increases mitochondrial mobility. (C) After massive synaptic and dendritic degeneration, these mitochondria end up in the axons where they could reverse the injury-induced energy deficit, thereby enabling growth cone formation and axonal regrowth.
Acknowledgments
The authors thank Annelies Van Dyck (Neural Circuit Development and Regeneration Research Group, Department of Biology, KU Leuven, Leuven, Belgium) for her helpful comments on the manuscript. Due to the strict reference limit, the authors regret citing studies without providing the associated references.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: This work was supported by a FWO (Fonds voor Wetenschappelijk Onderzoek) Flanders-Quebec bilateral research grant, a KU Leuven C1 grant (C14/18/053) and L’Oréal-UNESCO “For women in science” (FWO).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Arne M. Nystuen, Neurotech Pharmaceuticals, USA.
Funding: This work was supported by a FWO (Fonds voor Wetenschappelijk Onderzoek) Flanders-Quebec bilateral research grant, a KU Leuven C1 grant (C14/18/053) and L’Oréal-UNESCO “For women in science” (FWO).
P-Reviewer: Nystuen AM; C-Editors: Zhao M, Yu J; T-Editor: Liu XL
References
- 1.Agostinone J, Alarcon-Martinez L, Gamlin C, Yu WQ, Wong ROL, Di Polo A. Insulin signalling promotes dendrite and synapse regeneration and restores circuit function after axonal injury. Brain. 2018;141:1963–1980. doi: 10.1093/brain/awy142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beckers A, Van Dyck A, Bollaerts I, Van Houcke J, Lefevere E, Andries L, Agostinone J, Van Hove I, Di Polo A, Lemmens K, Moons L. An antagonistic axon-dendrite interplay enables efficient neuronal repair in the adult Zebrafish central nervous system. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1292-5. doi: 10.1007/s12035-018-1292-5. [DOI] [PubMed] [Google Scholar]
- 3.Cai Q, Sheng ZH. Mitochondrial transport and docking in axons. Exp Neurol. 2009;218:257–267. doi: 10.1016/j.expneurol.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cartoni R, Norsworthy MW, Bei F, Wang C, Li S, Zhang Y, Gabel CV, Schwarz TL, He Z. The mammalian-specific protein Armcx1 regulates mitochondrial transport during axon regeneration. Neuron. 2017;94:689. doi: 10.1016/j.neuron.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 5.Chung SH, Awal MR, Shay J, McLoed MM, Mazur E, Gabel CV. Novel DLK-independent neuronal regeneration in Caenorhabditis elegans shares links with activity-dependent ectopic outgrowth. Proc Natl Acad Sci U S A. 2016;113:E2852–2860. doi: 10.1073/pnas.1600564113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drummond ES, Rodger J, Penrose M, Robertson D, Hu Y, Harvey AR. Effects of intravitreal injection of a Rho-GTPase inhibitor (BA-210), or CNTF combined with an analogue of cAMP, on the dendritic morphology of regenerating retinal ganglion cells. Restor Neurol Neurosci. 2014;32:391–402. doi: 10.3233/RNN-130360. [DOI] [PubMed] [Google Scholar]
- 7.Han SM, Baig HS, Hammarlund M. Mitochondria localize to injured axons to support regeneration. Neuron. 2016;92:1308–1323. doi: 10.1016/j.neuron.2016.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kravtsov V, Oren-Suissa M, Podbilewicz B. The fusogen AFF-1 can rejuvenate the regenerative potential of adult dendritic trees by self-fusion. Development. 2017;144:2364–2374. doi: 10.1242/dev.150037. [DOI] [PubMed] [Google Scholar]
- 9.Mar FM, Simoes AR, Leite S, Morgado MM, Santos TE, Rodrigo IS, Teixeira CA, Misgeld T, Sousa MM. CNS axons globally increase axonal transport after peripheral conditioning. J Neurosci. 2014;34:5965–5970. doi: 10.1523/JNEUROSCI.4680-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinn TE, Mitchell DM, Meighan PC, Partington N, Leoni DC, Jenkins CE, Varnum MD, Stenkamp DL. Restoration of dendritic complexity, functional connectivity, and diversity of regenerated retinal bipolar neurons in adult Zebrafish. J Neurosci. 2018;38:120–136. doi: 10.1523/JNEUROSCI.3444-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paveliev M, Fenrich KK, Kislin M, Kuja-Panula J, Kulesskiy E, Varjosalo M, Kajander T, Mugantseva E, Ahonen-Bishopp A, Khiroug L, Kulesskaya N, Rougon G, Rauvala H. HB-GAM (pleiotrophin) reverses inhibition of neural regeneration by the CNS extracellular matrix. Sci Rep. 2016;6:33916. doi: 10.1038/srep33916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stone MC, Albertson RM, Chen L, Rolls MM. Dendrite injury triggers DLK-independent regeneration. Cell Rep. 2014;6:247–253. doi: 10.1016/j.celrep.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y, Chen M, Hu B, Huang R, Hu B. In vivo imaging of mitochondrial transport in single-axon regeneration of Zebrafish mauthner cells. Front Cell Neurosci. 2017;11:4. doi: 10.3389/fncel.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou B, Yu P, Lin MY, Sun T, Chen Y, Sheng ZH. Facilitation of axon regeneration by enhancing mitochondrial transport and rescuing energy deficits. J Cell Biol. 2016;214:103–119. doi: 10.1083/jcb.201605101. [DOI] [PMC free article] [PubMed] [Google Scholar]