Table 3. Pronucleophile scope for internal diene addition reactions a , b , c .
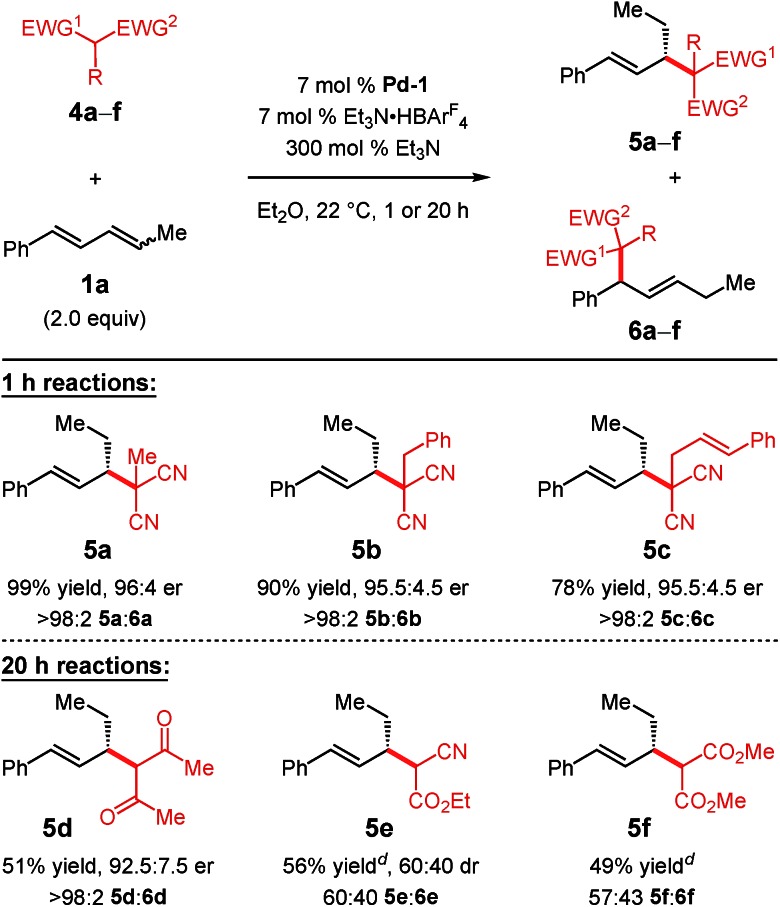
|
aReaction under N2 with 0.2 mmol pronucleophile in 0.2 mL Et2O. 1a used as a 1.8:1 E,Z : E,E mixture of stereoisomers.
bIsolated yield of purified product.
cer determined by HPLC analysis of purified 5.
dIsolated yield of the isomeric mixture; er not determined.
