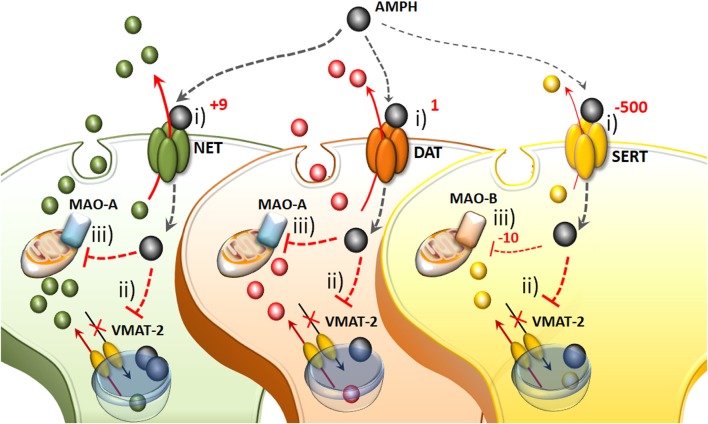
Figure 1.
The molecular mechanisms of amphetamine(s) (AMPHs) in monoamine-containing neurons. (i) The primary molecular target, which provides neuronal selectivity for AMPHs, consists in the plasma membrane transporter. In fact, AMPHs behave as competitive substrates for the re-uptake through the NE transporter (NET), dopamine (DA) transporter (DAT) and 5-HT transporter (SERT; Rothman and Baumann, 2003; Fleckenstein et al., 2007). These transporters normally work by taking up extracellular monoamines to the axoplasm, which is the main mechanism to terminate their activity (Iversen et al., 1965; Axelrod and Kopin, 1969; Coyle and Axelrod, 1971; Aggarwal and Mortensen, 2017). Cross-affinity between AMPHs and neurotransmitters contributes to generate the quite selective storage of AMPHs within specific neurons. Once bound to the plasma membrane transporter, AMPHs enter the axoplasm while reverting the transport direction (Sulzer et al., 1993). This occurs mostly for catecholamine neurons since AMPHs strongly discriminate between SERT, to which they bind with much lower affinity (500-fold less) compared with DAT and NET (Rothman and Baumann, 2003). In particular, AMPHs bind to the NET with five-to-nine-fold higher affinity compared with the DAT (Rothman and Baumann, 2003). This is the main reason why AMPHs release NE more potently than DA and much more than 5-HT (Rothman et al., 2001). (ii) Within monoamine axons, AMPHs encounter a second specific target called vesicular monoamine transporter type-2 (VMAT-2), which is also shared with monoamines. In this way, AMPHs enter the synaptic vesicles. At this level, AMPHs impair the acidification of the vesicle, which generates an acidic pH (Sulzer and Rayport, 1990; Sulzer et al., 1993, 1995). This acidic environment is erased by AMPHs, which rise the vesicular pH value from 4 up to 7, which corresponds to a 1,000-fold increase in the concentration of H+ ions. Thus, monoamines, which are weak bases, are charged at low pH, while at a neutral pH lose their charge, and diffuse through the vesicle membrane, thus massively invading the axoplasm (Brown et al., 2000, 2002; Pothos et al., 2000). In this way, axonal monoamines either passively or via a reverted plasma membrane transporter fill extracellular space where they reach a massive concentration (Sulzer et al., 1995, 2005). (iii) The third molecular target, which is impaired by AMPHs, is the mitochondrial-bound enzyme monoamine oxidase (MAO). Both MAO-A/-B iso-enzymes oxidatively deaminate DA, NE and 5-HT. Nonetheless, MAO-A/-B isoforms differ in substrate preference, inhibitor affinity and regional distribution within either single neurons or different animal species (Robinson et al., 1977; Youdim, 1980; Sourkes, 1983; Gesi et al., 2001; Youdim et al., 2006; Bortolato et al., 2008). These differences are seminal to explain the specific effects of AMPHs within various monoamine neurons. In fact, MAO-A, are competitively inhibited by methamphetamine (METH) with a 10-fold higher affinity compared with MAO-B. MAO-A is placed within synaptic terminals of DA and NE neurons, while MAO-B are the only isoform operating within 5-HT terminals and non-catecholamine neurons. Thus, apart from rats and a few animal species, the effect of AMPHs on the amount of extracellular monoamines is remarkable concerning NE and DA, being less pronounced for 5-HT.