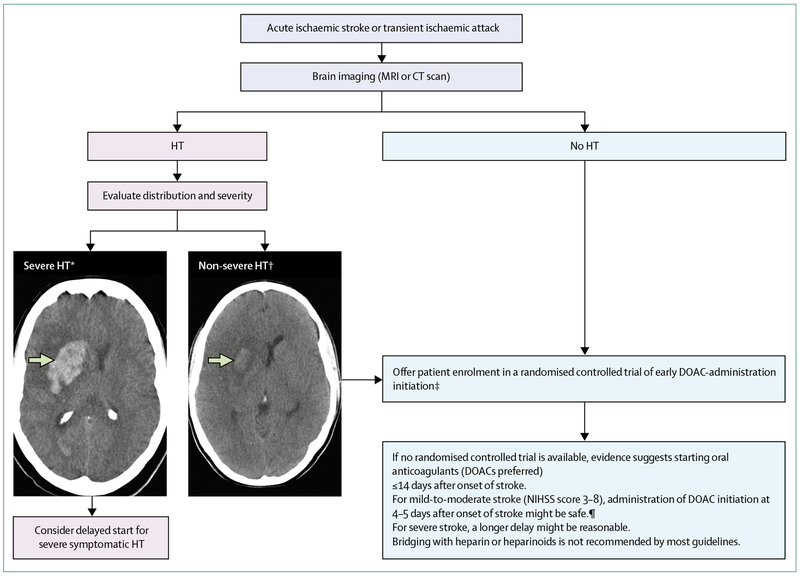
Figure 2: Timing for initiation of direct oral anticoagulant administration.
Enrolment of eligible patients with ischaemic stroke and atrial fibrillation in ongoing randomised controlled trials is suggested on the basis of current guidelines and evidence from observational studies and clinical trials. If enrolment in a trial is not possible, clinicians will need to use the data to weigh potential risks and benefits of early DOAC administration although evidence is currently scarce to make strong recommendations. DOAC=direct oral anticoagulant. HT=haemorrhagic transformation. NIHSS= National Institutes of Health Stroke Scale. *Parenchymal haematoma Type 2 (homogeneous hyperdensity with mass effect >30% of infarct volume or extending beyond the infarct). †Petechial haemorrhage Type 1 (petechial heterogeneous hyperdensity), petechial haemorrhage Type 2 (diffuse heterogeneous hyperdensity), or parenchymal haemorrhage Type 1 (homogeneous hyperdensity <30% of infarct).56 ‡If oral anticoagulants are not contraindicated. ¶Prospective observational data and one small randomised controlled trial; grade of recommendation B, level of evidence 2a.