Table 1. Reaction optimisation for iron- and cobalt-catalysed hydrosilylation using tetrafluoroborate pre-catalyst activation a .
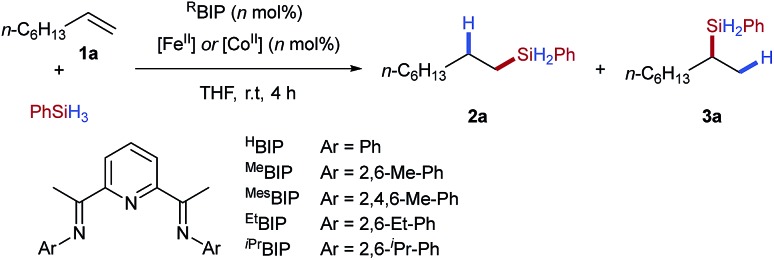
| ||||
| Entry | [M] | Loading (mol%) | Ligand | Yield (%) (2a : 3a) |
| 1 | FeCl2 | 2 | EtBIP | 0 |
| 2 | Fe(OTf)2 | 2 | EtBIP | 0 |
| 3 | Fe(BF 4 ) 2 ·6H 2 O | 2 | Et BIP | 87 (93 : 7) |
| 4 | Fe(BF4)2·6H2O | 0.5 | EtBIP | 67 |
| 5 | Fe(BF4)2·6H2O | 1 | EtBIP | 82 |
| 6 | Fe(BF4)2·6H2O | 2 | HBIP | 0 |
| 7 | Fe(BF4)2·6H2O | 2 | MeBIP | Trace |
| 8 | Fe(BF4)2·6H2O | 2 | MesBIP | 78 (>95 : 5) |
| 9 | Fe(BF4)2·6H2O | 2 | iPrBIP | Trace |
| 10 | Co(BF4)2·6H2O | 2 | HBIP | 84 (86 : 14) |
| 11 | Co(BF4)2·6H2O | 2 | MeBIP | 72 (4 : 96) |
| 12 | Co(BF4)2·6H2O | 2 | MesBIP | 68 (3 : 97) |
| 13 | Co(BF4)2·6H2O | 2 | EtBIP | 82 (5 : 95) |
| 14 | Co(BF4)2·6H2O | 2 | iPrBIP | 31 (16 : 84) |
| 15 | Co(BF 4 ) 2 ·6H 2 O | 1 | Et BIP | 90 (8 : 92) |
aReaction conditions: 1-octene (1.00 equiv.), phenylsilane (1.10 equiv.) and metal tetrafluoroborate (n mol%), THF (1 M), r.t., 1 h. Yields determined by 1H NMR spectroscopy of the crude reaction mixture using 1,3,5-trimethoxybenzene as an internal standard.
