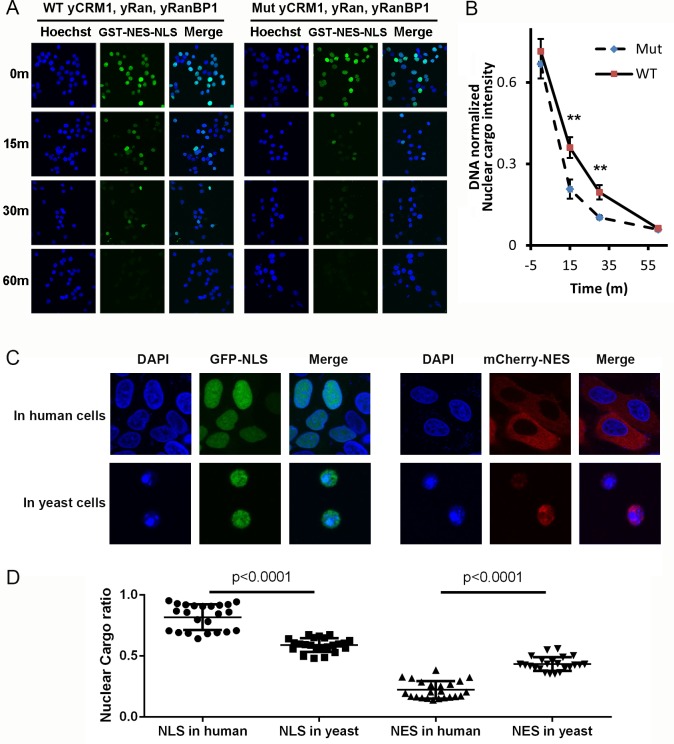
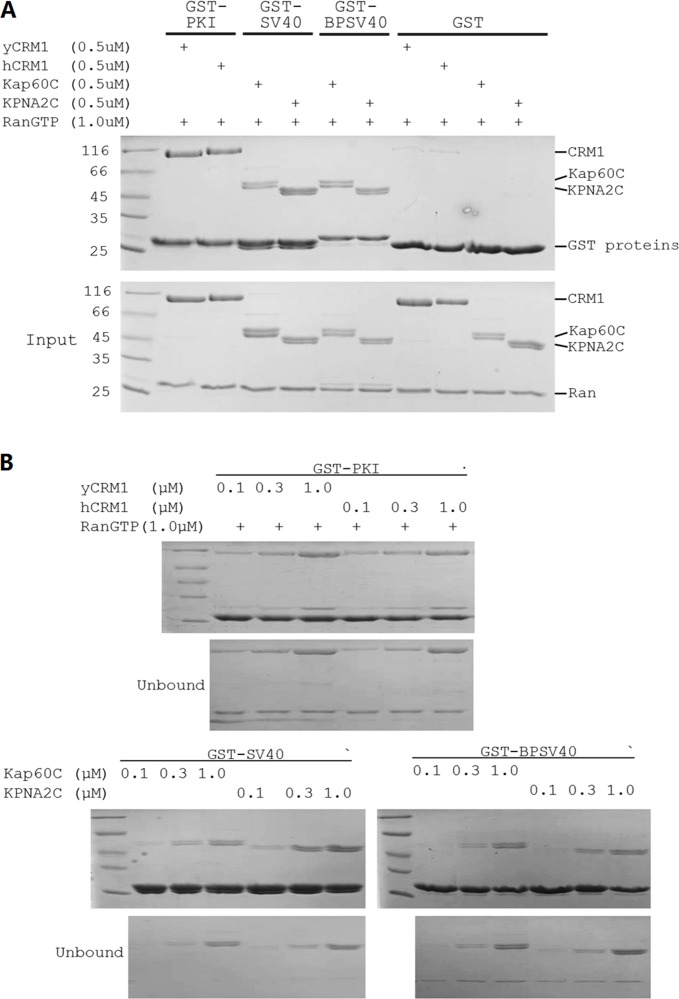
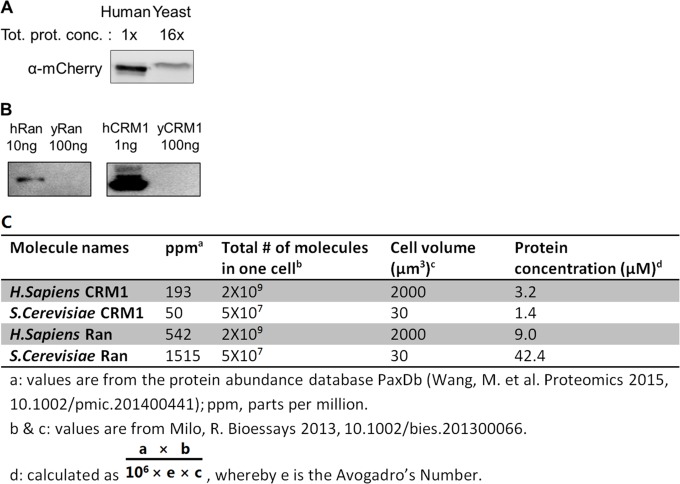
Figure 8. Mammalian nuclear transport system has higher efficiency.
(A) Nuclear export of GST-NES-NLS in the presence of yCRM1/yRan/yRanBP1 or their mutants (yCRM1T753Q, yRan, yRanBP1V150A and fused with mRanBP1’s NES). Protocol used in this experiment is similar as in Figure 3C. (B) Quantification of nuclear cargo intensity normalized by DNA in Figure 7E. Shown also includes unpaired student t-test between WT and mutant samples at each time point. Error bars represent standard error of measurements for each set of data containing measurements from at least 23 cells. Only 15 m and 30 m samples display statistical significances (** denotes p<0.01). (C) Representative images of GFP-NLS and mCherry-NES localization in human (HeLa) and yeast cells (W303.1a). pRS416 (for yeast transfection), pEGFP-C1 and pmCherry-C1 (for human transfection) plasmids were used to express fluorescent tagged NES or NLS proteins. mCherry-NES is constructed as mCherry-NESPKI-MBP-NLSSV40. GFP-NLS is constructed as GFP-NESPKI-MBP-NLSBPSV40. (D) Quantification and statistical analysis of nuclear cargo ratio in 8C.