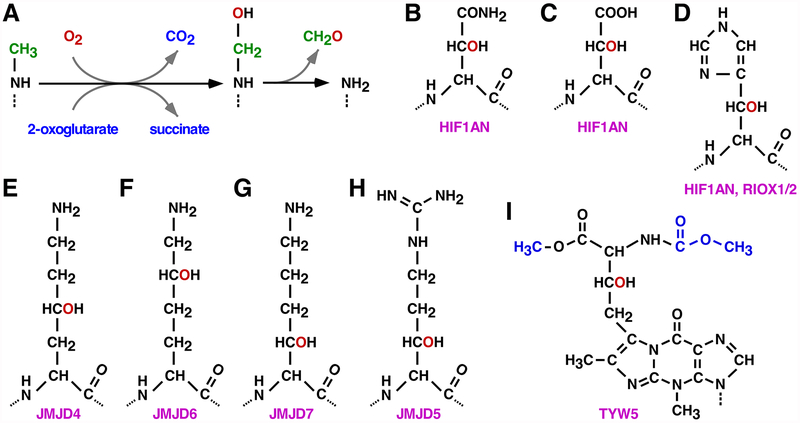
Fig. 2.
Oxygen- and 2OG-dependent catalytic activities displayed by JMJD proteins. (A) Demethylation of a monomethylated lysine residue. The first step is the hydroxylation of the methyl moiety, leading to a labile carbinolamine that spontaneously releases formaldehyde. Di- and trimethylated lysine residues are also utilized as substrates by several JMJD proteins. (B, C) Hydroxylation of an asparagine or aspartate residue by HIF1AN. (D) Hydroxylated histidine residue as a consequence of HIF1AN or RIOX1\2 catalytic activity. (E-G) Hydroxylation of lysine at the C4, C5 or C3 position by JMJD4, JMJD6 or JMJD7, respectively. (H) JMJD5- mediated hydroxylation at the C3 position of an arginine residue. (I) Hydroxywybutosine. Please note that TYW5 hydroxylates a precursor of wybutosine, after which TYW4 catalyzes the addition of further modifications (marked in blue color). In all panels, red color highlights the oxygen added upon JMJD catalytic activity.