Figure 3.
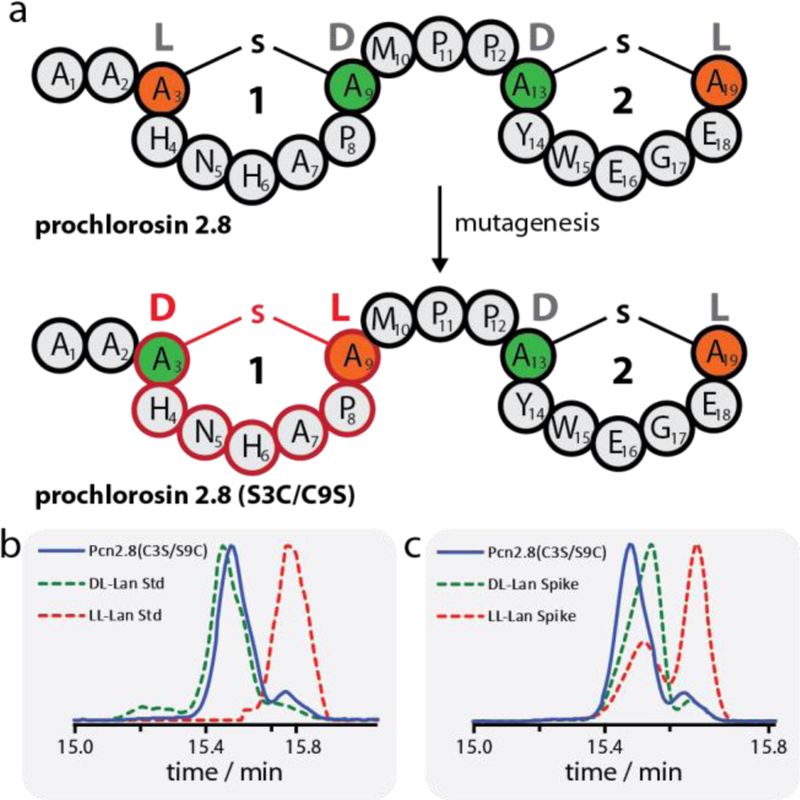
(a) Schematic representation of WT Pcn2.8 and Pcn2.8(S3C/C9S) demonstrating how mutagenesis can be used to invert the stereochemistry of ring 1 and thus yield a diastereomer of the WT lanthipeptide. Orange residues derive from Cys, green residues derive from Ser. (b-c) GC-MS analysis of the lanthionine bis-amino acids in Pcn2.8(C3S/S9C). Shown in (b) is the sample trace (blue) overlayed with traces of the DL-lanthionine (green dashed) and LL-lanthionine (red dashed) standards. Shown in (c) are the traces of the sample (blue) and sample spiked with either the DL-lanthionine (green dashed) or LL-lanthionine (red dashed) standard. The experiments clearly show that the lanthionine bis-amino acids of Pcn2.8(C3S/S9C) have DL stereochemistry. The acid hydrolysis of the peptide results in a small amount of epimerization as previously reported.50
