Abstract
Soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins mediate membrane fusion events in eukaryotic cells. Traditionally recognized as major players in regulating presynaptic neurotransmitter release, accumulative evidence over recent years has identified several SNARE proteins implicated in important postsynaptic processes such as neurotransmitter receptor trafficking and synaptic plasticity. Here we analyze the emerging data revealing this novel functional dimension for SNAREs with a focus on the molecular specialization of vesicular recycling and fusion in dendrites compared to those at axon terminals and its impact in synaptic transmission and plasticity.
Introduction
Membrane fusion is mediated by the formation of the SNARE complex assembly via the interaction of SNARE proteins. SNAREs are categorized in three families depending on their subcellular localizations. Synaptosomal-associated proteins (SNAP) and syntaxins (Stx) belong to the target SNARE (t-SNAREs) family and are located at target membranes. Synaptobrevin (Syb) (a.k.a VAMP, from vesicle-associated membrane protein) are vesicle SNAREs (v-SNARE) enriched in vesicle membranes. Syntaxin and synaptobrevins have a single copy of the conserved SNARE motif whereas SNAPs contribute with two copies. SNARE motif interaction forms tight four-helix helical bundles resistant to SDS (Hayashi et al., 1994; Sutton et al., 1998). The formation of the SNARE complex brings into close apposition the target and vesicle membranes during an exothermic process that overcomes the energy barrier required for membrane fusion (Südhof and Rothman, 2009).
Arguably, the most extensive studied example of SNARE-dependent fusion has been the presynaptic release of neurotransmitter (Südhof, 2008). The canonical SNARE complex in the presynaptic region is constituted by the association of synaptosomal-associated protein of 25 KDa (SNAP-25) in combination with Stx-1 and Syb-2/VAMP-2 (Jahn and Scheller, 2006; Rizo and Rosenmund, 2008; Südhof and Rothman, 2009). Calcium sensor proteins such as synaptotagmins are crucial for culminating calcium-dependent exocytosis (Fernández-Chacón et al., 2001; Südhof and Rothman, 2009; McMahon et al., 1995; Xu et al., 2007; Schonn et al., 2008; Cai et al., 2008) in a millisecond timescale. Interestingly, specific synaptotagmin isoforms have been found to be implicated in postsynaptic exocytosis during activity-dependent plasticity (Wu et al., 2017).
SNARE-dependent fusion is highly efficient thus it is unsurprising neurons employ similar machinery for different membrane fusion events including exocytic events in dendrites. At the postsynaptic compartment rapid changes in the composition of neurotransmitter receptors via active exo- and endocytosis processes regulate synaptic transmission and plasticity. In the mammalian brain, excitatory transmission is mediated by glutamate receptor activation, mostly AMPA and NMDA receptors (AMPAR and NMDAR) which are thought to undergo constitutive and activity-dependent trafficking. Membrane dynamics of AMPAR have been most extensively studied as an underlying mechanism of synaptic plasticity, a neuronal correlate of learning and memory (Malenka and Bear, 2004). Robust activation of calcium-permeable NMDAR often triggers AMPAR insertion into the plasma membrane thus eliciting long-term synaptic potentiation (LTP) (Bredt and Nicoll, 2003; Collingridge et al., 2004; Malinow and Malenka, 2002; Newpher and Ehlers, 2008; Shepherd and Huganir, 2007). AMPARs may reach synapses from perisynaptic sites (Lu et al., 2007; Petrini et al., 2009) from where they can laterally diffuse into the postsynaptic density (PSD) where are stabilized by synaptic scaffolds like PSD-95 (Henley et al., 2011; Kennedy and Ehlers, 2011; Opazo and Choquet, 2011). In the other hand, mild stimulation of NMDARs regularly yields long-term depression (LTD) of synaptic transmission which involves plasma membrane removal of AMPARs (Malenka and Bear, 2004). The elucidation of the mechanisms involved in AMPAR trafficking is crucial given the role of synaptic plasticity in experience-dependent plasticity (Malenka and Bear, 2004; Neves et al., 2008), and as a neuronal property that appears impaired in numerous neuropsychiatric and neurological disorders (Clapp et al., 2012; Ehlers, 2012).
Despite dendritic fusion events are usually triggered by elevation of calcium in the cytosol, there are important differences between pre- and postsynaptic exocytosis. For once, presynaptic terminals store multiple synaptic vesicles which are docked and primed at the plasma membrane which allows ultra-fast fusion and release upon calcium entry. In contrast, docked or primed postsynaptic vesicles have not been shown. For example, AMPAR-containing endosomes are not accumulated close to the plasma membrane. Instead of being stored at defined regions nearby the plasma membrane, AMPAR-containing endosomes move along the dendrite through a myosin-dependent mechanism (Correia et al., 2008; Wang et al., 2008). The lack of an active zone-like region in dendrites may contribute to the fact that AMPARs insertion events in response to NMDAR activation occurs in a time scale of several seconds or even minutes (Patterson et al., 2010; Petrini et al., 2009; Yang et al., 2008; Yudowski et al., 2007). Distinctive properties of postsynaptic release may be likely explained by the involvement of specific SNARE proteins. The composition of the postsynaptic SNARE complexes mediating synaptic transmission and plasticity has just begun to be revealed. Here, we review recent findings on postsynaptic SNARE proteins involved in neurotransmitter receptor trafficking. These new data suggest molecularly distinct SNARE complexes regulate different postsynaptic trafficking pathways providing highly selective control of synaptic transmission and plasticity.
Postsynaptic SNARE proteins in synaptic transmission and plasticity
Role of postsynaptic synaptobrevins/VAMPs
Synaptobrevins are small transmembrane proteins located at vesicle membranes (Ernst and Brunger, 2003; Brunger et al., 2009). The main brain isoforms are Syb-1 and Syb-2, with Syb-2 being much more prominent in the forebrain than Syb-1 which is more abundant in the spinal cord and neuromuscular junctions (Trimble et al., 1990; Elferink et al., 1989; Raptis et al., 2005). Despite being closely related to Syb-2, Syb-1 is considered to be insensitive to tetanus toxin (TeTx) and botulinum toxin serotype B (BoNTB) which specifically cleaves Syb-2 (Schiavo et al., 2000).
During early development Syb-2 can be found in dendrites due to transcytosis (Sampo et al., 2003; Wisco et al., 2003; Yap et al., 2008; Ascaño et al., 2009) suggesting that besides being an integral molecule of synaptic vesicles Syb-2 reaches postsynaptic membrane compartments. Furthermore, analysis of subcellular expression using western blot and immunogold have revealed Syb-2 in postsynaptic compartments in the CA1 hippocampus of adult rats where it appears associated with AMPAR-containing vesicles, particularly those containing the GluA1 subunit (Hussain and Davanger, 2015). In addition to its presence at AMPAR-containing endosomes, several studies have shown the involvement of Syb-2 in regulating AMPAR trafficking. In a seminal paper, Lledó and colleagues (1998) successfully disrupted LTP by postsynaptically infusing BoNTB. Further, spine growth associated with synaptic potentiation is also blocked by BoNTB (Murakoshi and Yasuda, 2012) suggesting that a Syb-2-containing SNARE complex is required for both AMPAR exocytosis during synaptic potentiation and spine growth.
More recently, experiments in cultures prepared from synaptobrevin-2 KO mice indicated that Syb-2 contributes to maintaining both synaptic and extrasynaptic GluA1-containing AMPARs (Jurado et al., 2013) (Figure 1). According with this idea, a recent report from Husain and Davanger (2015) has shown how Syb-2 cleavage with TeTx impairs GluA1-containing AMPARs in the postsynaptic membrane. In addition, a potential role of Syb-2 in constitutive trafficking has been hinted in a recent study showing that Syb-2 also participates in the insertion of GluA2-containing receptors (Araki et al., 2010). These results suggest Syb-2 may be an integral element of AMPAR-containing endosomes participating in the regulation of both constitutive and activity-dependent trafficking pathways. Furthermore, Syb-2 has also been involved in AMPAR insertion during homeostatic potentiation induced by retinoic acid (Arendt et al., 2015) and even in the exocytosis of GABAARs in hippocampal neurons (Gu et al., 2016) (Figure 1). These findings suggest that similarly to synaptic vesicles (Takamori et al., 2006), Syb-2 is an abundant element of postsynaptic vesicles and thus participates in different dendritic trafficking processes.
Figure 1: Functions of postsynaptic synaptobrevins.
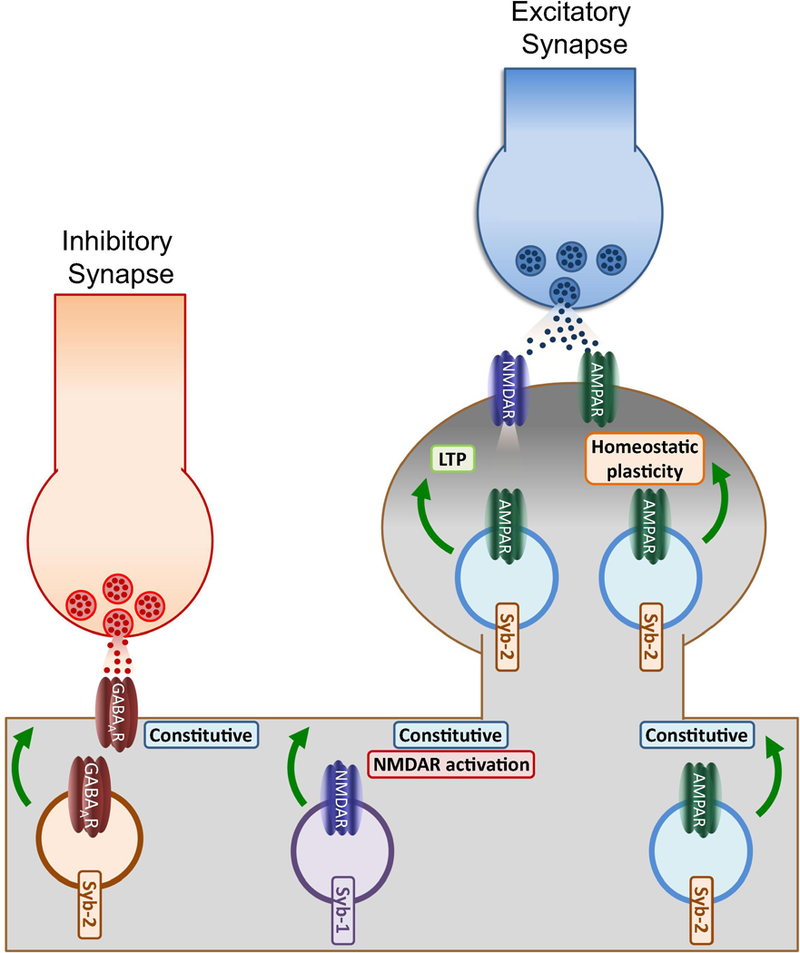
The model summarizes some of our current knowledge of the role of postsynaptic Sybs in regulating neurotransmitter receptor trafficking. Syb-2 has been suggested to be an integral part of GABAAR-containing vesicles and regulate basal insertion in dendritic membranes. Moreover, Syb-2 may be located at AMPAR-containing endosomes, thus participating in basal and activity-dependent exocytic events either induced by NMDAR activation or during synaptic scaling induced by retinoic acid, a type of homeostatic plasticity. In addition, Syb-1, another common isoform of Syb in the CNS, has been suggested to specifically regulate constitutive NMDAR exocytosis.
However, some important postsynaptic functions may be taken over by Syb-1. Whereas BoNTB treatment effectively blocks AMPAR insertion during LTP, it does not affect NMDAR function (Lledó et al., 1998; Lüscher et al., 1999). Likewise TeTx does not prevent the enhancement of NMDAR-mediated currents during glycine-induced potentiation (Lu et al., 2001). These findings suggest NMDAR insertion is mediated by a Syb isoform insensitive to BoNTB such as Syb-1. This seems to be the case for constitutive trafficking of NMDARs which has been recently shown to be significantly impaired in response to a specific Syb-1 knockdown (Gu and Huganir, 2016) (Figure 1). These results suggest different Syb isoforms regulate specific trafficking pathways in dendrites.
Role of postsynaptic syntaxins
Syntaxins are small transmembrane proteins that comprised a family of 15 members (Advani et al., 1998) from which only four isoforms (Stxs 1–4) localize to the plasma membrane where they mediate membrane fusion events (Bennett et al., 1993; Gaisano et al., 1996). The two isoforms of Stx-1 (Stx-1a and Stx-1b) are the most heavily studied due to their fundamental role in controlling vesicle docking and exocytosis of presynaptic vesicles (Jahn and Südhof, 1999). According to its function, Stx-1 is mainly expressed in presynaptic compartments (Stx-1a constitutes ~ 1% of all brain protein; Lang and Jahn, 2008) whereas Stxs 2, 3, and 4 have a wider tissue distribution (Bennett et al., 1993). Syntaxins cluster into membrane microdomains which may serve as hot spots for exocytosis. The formation of syntaxin-enriched microdomains in the dendrite plasma membrane may serve as a signal for favorable regions of exocytosis that otherwise would go unnoticed in the absence of a structured molecular scaffold characteristic of the presynaptic active zone (Lang et al., 2001; Ohara-Imaizumi et al., 2004; Low et al., 2006; Sieber et al., 2007; Kennedy et al., 2010). Interestingly, complexin a protein critical for calcium-dependent exocytosis (Takahashi et al., 1999; Huang et al., 2000) has been shown implicated in dendritic fusion during LTP (Ahmad et al., 2012). Moreover, structure-analysis experiments shown that complexin requires the interaction with SNARE proteins to regulate LTP (Ahmad et al., 2012) suggesting the presence of a postsynaptic Stx capable of binding complexin such as Stx-1 and Stx-3 (Pabst et al., 2000). Here we will focus on the postsynaptic function of Stxs 1, 3 and 4 as currently there are no reports investigating the role of Stx-2 in synaptic transmission and plasticity.
Postsynaptic syntaxin-1
According to their function in regulating neurotransmitter release, Stx-1 is predominantly localized at presynaptic plasma membranes (Koh et al., 1993). However a recent analysis using electron microscopy and quantitative post-embedding immunogold has revealed Stx-1 in dendritic spines (Hussain et al., 2016). Additional experiments of immunofluorescence double labeling with PSD-95 and ultrastructural fractionation of synaptosomes also confirmed Stx-1 localization at the PSD (Hussain et al., 2016). Intriguingly, postsynaptic Stx-1 strongly colocalized with the GluN2B subunit of NMDARs suggesting a potential role in NMDAR trafficking. This role may be subunit specific as the insertion of the GluN2A subunit has been reported to depend on Stx-4 (Gu and Huganir, 2016). By affecting GluN2B trafficking, Stx-1 may contribute to shape NMDAR-mediated currents and synaptic plasticity. Conversely, specific postsynaptic knockdown targeted to both Stx-1a and Stx-1b did not affect LTP in hippocampal slices (Jurado et al., 2013) implying that reducing endogenous Stx-1 in the postsynapse does not alter NMDAR function enough to block synaptic potentiation. Nevertheless, changes in NMDAR subunit composition such swapping GluN2B for GluN2A during development (Liu et al., 2004), are known to modify NMDAR function in normal and pathological conditions (Sprengel et al., 1998; Zhou and Baudry, 2006). Therefore a role of postsynaptic Stx-1 in specifically regulating GluN2B trafficking may be fundamental for maintaining normal NMDAR-mediated transmission. In addition to regulate glutamate receptor trafficking, a specific knockdown targeting both Stx-1a and Stx-1b has been shown to significantly reduce the plasma insertion frequency of γ2-containing GABAARs in rat hippocampal neurons (Gu et al., 2016) suggesting a central role for postsynaptic Stx-1 in regulating constitutive trafficking of excitatory and inhibitory receptors contributing to the basal excitatory/inhibitory balance (Figure 2).
Figure 2: Functions of postsynaptic syntaxins.
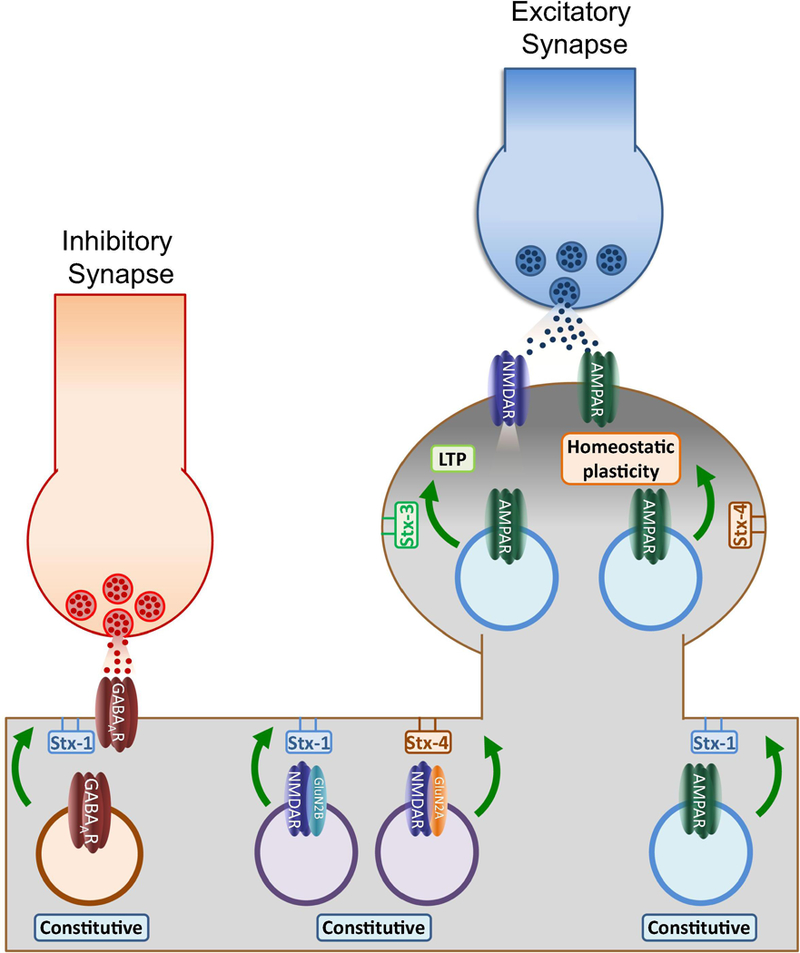
The model represents the role of different postsynaptic syntaxins in regulating neurotransmitter receptor dynamics. Despite being mostly concentrated at presynaptic terminals, Stx-1 has been suggested to play a role in regulating constitutive exocytosis of GABAARs, AMPARs and GluN2B-containing NMDA receptors. Interestingly, GluN2A-containing NMDARs insertion may depend on Stx-4, a non-complexin Stx isoform that also mediates AMPAR exocytosis during homeostatic plasticity. In contrast Stx-3, a complexin-binding isoform is the primary regulator of AMPAR exocytosis during NMDAR-dependent LTP. These results suggest dendritic membranes may contain microdomains enriched with different Stx isoforms which define specific regions for postsynaptic receptor exocytosis.
Postsynaptic syntaxin-3
Stx-3 has been recently proposed to control AMPARs exocytosis during LTP through its interaction with complexin (Jurado et al., 2013). In vivo stereotaxic injections of a specific shRNA against endogenous Stx-3 dramatically impaired LTP in acute hippocampal slices without affecting neither basal transmission nor presynaptic properties (Figure 2). Moreover, the same Stx-3 shRNA effectively blocked glycine-dependent LTP in cultured hippocampal neurons and both forms of plasticity were fully rescued by restoring Stx-3 levels (Jurado et al., 2013). Furthermore, similarly to presynaptic Stx-1, Stx-3 requires the binding to SM proteins which promote a conformational change that frees the SNARE motif to interact with the rest of the SNARE proteins (Jurado et al., 2013). According to its postsynaptic function in plasticity, Stx-3 has been shown to localize widespread throughout neurons where it can be found in both dendrites and axons (Jurado et al., 2013). Interestingly, Stx-3 may play a quite distinct role in NMDAR-dependent plasticity by specifically mobilizing AMPARs since the same Stx-3 shRNA does not affect other forms of plasticity like (Arendt et al., 2015) or other type of glutamate receptors like NMDARs (Jurado et al., 2013).
Postsynaptic syntaxin-4
Stx-4 also localizes to the plasma membrane but in contrast to Stx-1 and Stx-3 lacks the 12 aa stretch required for complexin interaction (Pabst et al., 2000). Stx-4 has been suggested to play a role in activity-dependent AMPAR exocytosis (Kennedy et al., 2010; Arendt et al., 2015) and NMDAR constitutive trafficking (Gu and Huganir, 2016) (Figure 2). In addition, a recent study using a Stx-4 KO model revealed a significant reduction of basal glutamatergic transmission due to a reduction of AMPARs and NMDARs (Bin et al., 2018). A function of Stx-4 in AMPAR insertion during LTP-like conditions in cultured neurons was first suggested by Kennedy et al., (2010). These authors observed a block of recycling endosome exocytosis in the presence of a specific Stx-4 shRNA. These results are conflicting with the findings suggesting the role of complexin in LTP (Ahmad et al., 2012). This apparent discrepancy may in large part be explained by the differences in the methods used to assay the fusion of AMPARs-containing endosomes. In Kennedy et al., (2010), recycling endosomes were detected with super ecliptic pHluorin (SEP)-fused transferrin receptors as a proxy to assay AMPAR-containing endosomes which may not accurately reflect the behavior of endogenous AMPARs like those assayed by electrophysiology. In addition, it may be plausible that Stx-4 may affect AMPAR exocytosis during LTP indirectly via affecting basal levels of NMDARs (Gu and Huganir, 2016). Another way Stx-4 may affect synaptic transmission may be through a recently appreciated role in modulating transynaptic adhesion molecules such as neuroligins (Harris et al., 2016) which are important for synapse formation and function (Südhof, 2008). However, a recent report using immunogold labeling showed endogenous Stx-4 primarily located on the plasma membrane of astrocytes in mixed neuronal cultures (Tao-Cheng et al., 2015) suggesting Stx-4 may play a role in glial exocytosis and thus impacting synaptic transmission indirectly.
Nonetheless, these results raise the intriguing possibility that different Stx isoforms may coexist in postsynaptic compartments and sort different cargos via independent microdomains (Puthenveedu et al., 2010; Temkin et al., 2011). Indeed, Stx-4 has been implicated in other forms of synaptic plasticity such as retinoic acid-induced potentiation (Arendt et al., 2015). These results suggest a potential model for alternative pathways of AMPAR exocytosis in where different postsynaptic Stxs define specific exocytic sites on the postsynaptic plasma membrane. Thus during LTP, AMPAR-containing vesicles may interact with complexin which interacts with membrane microdomains enriched with Stx-3, whereas during retinoic acid-dependent plasticity AMPAR-containing vesicles non-associated to complexin may dock and be released at Stx-4 microdomains.
Role of postsynaptic SNAPs
Postsynaptic SNAP-25
SNAP proteins are Q-SNARE proteins which contribute two SNARE motifs to complete the coiled-coil assembly required for the formation of the functional SNARE complex. There are four isoforms of SNAP proteins named according to their molecular weight: SNAP-23, SNAP-25, SNAP-29 and SNAP-47, being SNAP-25 the most heavily studied due to its principal role in neurotransmitter release. SNAP-25 deletion or cleavage by specific botulinum toxins abolishes synaptic transmission without significantly affecting vesicle docking (Sørensen et al., 2003; Pantano and Montecucco, 2014). SNAP-25 also interacts with VGCCs (Catterall and Few, 2008) negatively regulating calcium sensitivity to neuronal activation (Verderio et al., 2004; Condliffe et al., 2010). In addition to its role in exocytosis, SNAP-25 contributes to clathrin-dependent endocytosis (Zhang et al., 2013) and interacts with the endocytic protein intersectin (Okamoto et al., 1999) suggesting a role in maintaining the endo and exocytosis balance in neurons.
A postsynaptic role for SNAP-25 is primarily supported by functional studies since the expression of SNAP-25 at the postsynaptic compartment remains to be definitely probed (Holderith et al., 2012; Kerti et al., 2012; Suh et al., 2010, but also see Jordan et al., 2006; Selak et al., 2009). Manipulations targeted to impair SNAP-25 function impact glutamatergic synaptic transmission by disrupting actin cytoskeleton and spine formation (Tomasoni et al., 2013; Fossati et al., 2015). Acute in vivo knockdown of SNAP-25 has been shown to reduce the amount of mature dendritic spines in the CA1 region of the hippocampus (Tomasoni et al., 2013). In agreement with these data, SNAP-25 heterozygous mice show reduced levels of dendritic spines and altered PSD-95 trafficking (Fossati et al., 2015). In turn, SNAP-25 over-expression restores mature dendritic spines identified by the expression of the synaptic scaffold PSD-95. SNAP-25 regulates spine formation through an interaction with the plasma membrane and the adaptor protein p140Cap, which also interacts with PSD-95. These data suggest postsynaptic SNAP-25 regulates PSD-95 clustering which in turn may affect synaptic architecture and function (Fossati et al., 2015). These results may be especially relevant for neuropsychiatric disorders like schizophrenia in which significant reductions of synaptic proteins such as SNAP-25 has been described (Yin et al., 2012).
Moreover, SNAP-25 plays a role in kainate receptors (KARs) and NMDARs dynamics (Selak et al., 2009; Lau et al., 2010; Jurado et al., 2013). SNAP-25 promotes the endocytosis of GluK5 subunit of KARs through a PICK1-dependent mechanisms (Selak et al., 2009), whereas the PKC-mediated phosphorylation of SNAP-25 on S187, favors NMDAR insertion to the plasma membrane via SNARE-dependent exocytosis (Lau et al., 2010; Jurado et al., 2013). Accordingly, in vivo knockdown of SNAP-25 impairs LTP in hippocampal synapses as a result of decreasing synaptic NMDARs required for triggering synaptic plasticity (Jurado et al., 2013). Interestingly, SNAP-25 may affect NMDAR constitutive trafficking without affecting basal levels of AMPARs (Jurado et al., 2013; Suh et al., 2010; Gu and Huganir, 2016 but see also Gu et al., 2016) suggesting specific endosomal pathways may coexist for sorting different types of glutamatergic receptors (Figure 3) (Fong et al., 2002; Washbourne et al., 2002; Jeyifous et al., 2009).
Figure 3: Functions of postsynaptic SNAPs.
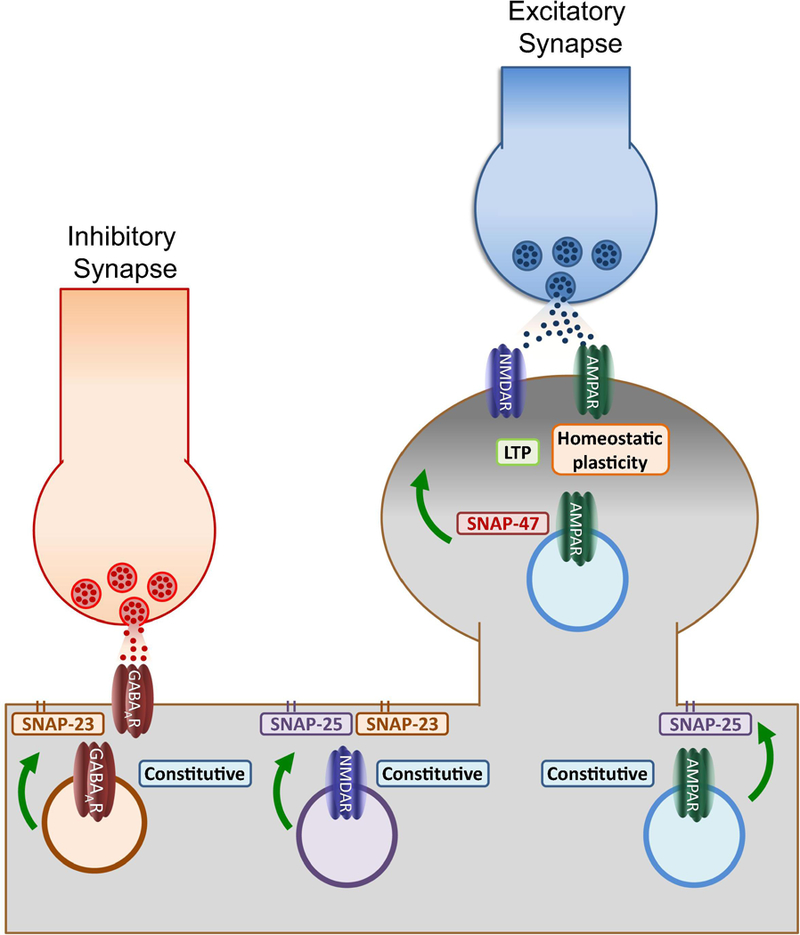
Schematic of the identified roles of postsynaptic SNAPs depicts membrane-bound SNAP-25 and SNAP-23 primarily involved in regulating constitutive trafficking of GABAA, AMPA and NMDA receptors. This function suggests the existence of specific membrane microdomains enriched with these SNAPs where exocytosis may be facilitated during baseline conditions. In contrast, activity-dependent insertion (NMDAR-dependent LTP and retinoic acid-induced potentiation) depends on the activity of SNAP-47, a broadly expressed cytosolic isoform. These results suggest that calcium influx increases dendritic exocytosis by recruiting SNAP isoforms like SNAP-47 which by not being attached to the membrane may define temporary regions for fusion along the dendritic membrane. By recruiting SNAP-47, the increase in postsynaptic exocytosis demand during synaptic plasticity can be supported in the absence of specialized regions like the presynaptic active zone.
Postsynaptic SNAP-23
Although first described as a SNAP-25 homolog in non-neuronal tissue (Ravichandran et al., 1996), later evidence indicated SNAP-23 is also expressed in the CNS (Chen et al., 1999; Verdeiro et al., 2004). SNAP-23 can functionally replace SNAP-25 in in vitro studies, however SNAP-23-containing SNARE complexes are less stable than those constituted by SNAP-25 (Vaidyanathan et al., 2001). Nonetheless SNAP-23 is capable of sustaining important secretion processes such as insulin exocytosis (Sadoul et al., 1997). SNAP-23 has been suggested to play a role in both GABAA and NMDA receptor trafficking (Gu et al., 2016; Suh et al., 2010). Using a heterozygous SNAP-23 knock-out mouse in combination with knockdown strategies, Suh et al., 2010 showed that reducing endogenous levels of SNAP-23 significantly decreases NMDAR surface expression and NMDAR-mediated synaptic transmission in hippocampal neuronal cultures and organotypic slices. Despite affecting NMDAR constitutive trafficking in cultured conditions, reducing endogenous SNAP-23 with a specific shRNA in vivo failed to impaired NMDAR-dependent LTP (Jurado et al., 2013) suggesting that either NMDAR trafficking relies more heavily on SNAP-23 during early stages of hippocampal development or that the remaining synaptic NMDARs are sufficient to support LTP induction in response to robust induction protocols commonly used in hippocampal slices.
In addition to glutamate receptor trafficking, SNAP-23 has also been proposed to participate in GABAAR dynamics (Gu et al., 2016) (Figure 3). Analysis of the insertion events corresponding to pHluorin-tagged AMPAR and GABAAR subunits (GluA2 and γ2, respectively) in the presence of a specific shRNA targeted to SNAP-23 has revealed that whereas SNAP-23 deletion does not impact constitutive AMPAR trafficking, surface expression of γ2-containing GABAAR and miniature IPSC amplitude appear significantly reduced in hippocampal neurons. These results again suggest specific roles of different postsynaptic SNARE proteins in regulating alternative sorting pathways for neurotransmitter receptors in neurons.
Postsynaptic SNAP-47
SNAP-47 is the most recent addition to the SNAP protein family and also the heaviest due to a long N-terminal stretch and an extended loop between its two SNARE motifs (Holt et al., 2006; Takamori et al., 2006). Similarly to SNAP-25, SNAP-47 is highly expressed in the CNS where it can be found in both axonal and dendritic compartments (Jurado et al., 2013; Shimojo et al., 2015; Münster-Wandowski et al., 2017). Nonetheless, SNAP-47´s role in exocytosis is unclear due to the formation of labile interactions with other SNARE proteins and the lack of palmitoylated cysteine clusters, important for the localization to surface membranes (Holt et al., 2006; Kuster et al., 2015). Nonetheless, the widespread expression of SNAP-47 throughout different subcellular compartments suggests a role in trafficking and membrane fusion (Holt et al., 2006). Indeed, SNAP-47 has been shown to play a function in activity-dependent AMPAR exocytosis (Jurado et al., 2013; Arendt et al., 2015) (Figure 3). In vivo SNAP-47 knockdown in the CA1 region abolishes LTP without affecting presynaptic properties or constitutive AMPAR/NMDAR trafficking highlighting a specific role of this protein in activity-dependent insertion of AMPARs (Jurado et al., 2013). In addition to this form of plasticity, SNAP-47 participates in AMPAR exocytosis during retinoic acid-induced homeostatic plasticity in hippocampal organotypic slices (Arendt et al., 2015). The weak interactions with other SNARE proteins (Holt et al., 2006) and the lack of membrane anchor motifs may contribute to make SNAP-47 the SNAP of choice for regulating exocytosis events during LTP which seem to occur randomly throughout the postsynaptic membrane (Yudowski et al., 2007; Petrini et al., 2009; Yang et al., 2008; Patterson et al., 2010).
Postsynaptic SNAP-29
SNAP-29 is an unconventional SNAP protein which similarly to SNAP-47, lacks palmitoylation sites for membrane anchoring (Steegmaier et al., 1998). In addition, SNAP-29 contains an N-terminal motif that facilities the interaction with endocytic adaptors and small GTPases like Rab3A (Rotem-Yehudar et al., 2001; Schardt et al., 2001). In contrast with SNAP-25 and SNAP-23, SNAP-29 has been reported to have a widespread expression throughout neurons and to interact with a wide variety of syntaxin isoforms (Steegmaier et al., 1998; Hohenstein and Roche, 2001). SNAP-29 levels in the CNS are low in comparison with its paralogs, however it has been reported that SNAP-29 mRNA expression may be regulated by electrical activity, such as a single session of electroconvulsive seizures on the rat frontal cortex induced an upregulation of the SNAP-29 messenger in the hippocampus (Elfving et al., 2008). The role of SNAP-29 in fusion events is controversial and its function in brain synaptic transmission and plasticity has not yet been explored. Some evidence suggests SNAP-29 can participate in plasma membrane fusion events whereas other studies restrict its function to regulating Golgi membrane dynamics (Wong et al., 1999). However, expression of SNAP-29 into presynaptic superior cervical ganglion neurons in culture significantly inhibited synaptic transmission in an activity-dependent manner (Su et al., 2001). Although we still ignore the function of SNAP-29 in the CNS there is some evidence suggesting alterations of the snap29 gene may be linked to neurological disorders such as schizophrenia and neuropathy associated with autophagy dysfunction (Saito et al., 2001; Morelli et al., 2014).
Concluding Remarks
Formation of SNARE complexes is a universal mechanism for membrane fusion and vesicle exocytosis. This efficient machinery regulates multiple neuronal functions being the most studied the presynaptic neurotransmitter release. However, evidence accumulated in recent years is exposing the role of SNAREs in regulating postsynaptic processes such as neurotransmitter receptor trafficking, thus expanding their role of master regulators of synaptic transmission and plasticity. Nonetheless, postsynaptic exocytosis exhibit different properties than presynaptic neurotransmitter release which are likely supported by molecularly distinct SNARE complexes. Whereas the canonical presynaptic SNARE complex is constituted by SNAP-25, Stx-1 and Syb-2, different postsynaptic forms of exocytosis seem to depend on specific SNARE proteins. For example, AMPAR insertion during LTP may recruit a complex formed by SNAP-47, Syb-2 and Stx-3 whereas receptors mobilized during retinoic acid-induced plasticity will be attracted to the membrane through a similar complex in which Stx-3 is replaced by Stx-4. In addition, constitutive NMDARs may require the formation of a SNARE complex constituted by SNAP-25 (which may substituted by SNAP-23), Syb-1 and Stx-4. Additionally, exocytosis of GABAAR-containing endosomes may preferentially recruit a SNARE complex formed by SNAP-23, Syb-2 and Stx-1. These findings suggest specific exocytic pathways coexist in dendrites in order to regulate neurotransmitter receptor insertion into the plasma membrane. Furthermore, the involvement of different Stxs opens the possibility that syntaxin-enriched microdomains mark specific regions of exocytosis for different neurotransmitter receptors.
Also intriguing is the fact that some secretion events at both the presynaptic and postsynaptic compartments seem to require the participation of the same SNARE molecules like Syb-2, thus only the unique combination of SNARE molecules mediates specific exocytic events. How do neurons regulate the trafficking of SNARE molecules to maintain adequate levels to support presynaptic neurotransmitter release and postsynaptic receptor levels in dendrites? Understanding these questions will provide insight not only into the details of synaptic transmission, but also general principles of how cellular subdomains are specified.
Moreover, calcium-dependent exocytosis such as presynaptic release and postsynaptic LTP require the participation of calcium binding proteins like synaptotagmin (Fernández-Chacón et al., 2001; Südhof and Rothman, 2009; McMahon et al., 1995; Xu et al., 2007; Schonn et al., 2008; Cai et al., 2008). Indeed, a role of complexin in LTP has been exposed (Ahmad et al., 2012) suggesting the participation of a postsynaptic synaptotagmin. Interestingly, synaptotagmin-1, the major trigger of fast neurotransmitter release, is not required for LTP (Ahmad et al., 2012). However simultaneous ablation of both synaptotagmin-1 and −7, a calcium sensor for short-term facilitation (Jackman et al., 2016), blocks LTP without affecting basal synaptic transmission (Wu et al., 2017). These findings suggest postsynaptic synaptotagmin-1 and −7 can play overlapping roles in AMPAR exocytosis during LTP.
Future efforts to elucidate the detailed molecular mechanisms involved in postsynaptic SNARE-dependent exocytosis involved in both synaptic transmission and plasticity will be critical for understanding the neural basis of many aspects of normal and pathological brain function.
Acknowledgments
Our laboratory is supported by funds from the National Institute of Aging (NIH, USA; RO1AG049937), the NARSAD Foundation (Young Investigator Award, 22688) and the Spanish State Research Agency, through the “Severo Ochoa” Programme for Centres of Excellence in R&D (SEV-2013–0317).
Abbreviations
- Aa
aminoacid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- BoNTB
botulinum toxin serotype B
- CA1
cornu ammonis 1
- CNS
central nervous system
- GluA1-A2
AMPA receptor subunit A1-A2
- GluN2A-2B
glutamate receptor subunit 2A-2B
- GABAAR
γ-aminobutyric acid receptor subunit A
- GluK5
glutamate ionotropic receptor kainate type subunit 5
- IPSC
inhibitory post-synaptic currents
- KARs
kainic acid receptors
- LTD
long-term depression
- LTP
long-term potentiation
- NMDAR
N-Methyl-D-aspartic receptor
- PSD-95
postsynaptic density-95
- PICK-1
protein interacting with C kinase 1
- Rab3A
ras-related protein 3A
- shRNA
short hairpin RNA
- SDS
sodium dodecyl sulfate
- SM
Sec1/Munc18-like
- SNARE
Soluble N-ethylmaleimide sensitive factor attachment protein receptor
- SNAP
synaptosomal-associated proteins
- Stx
syntaxin
- Syb
synaptobrevin
- TeTx
tetanus toxin
- VAMP
vesicle-associated membrane protein
- VGCC
voltage-gated calcium channels
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH (1998), Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem 273:10317–10324 [DOI] [PubMed] [Google Scholar]
- Ahmad M, Polepalli JS, Goswami D, Yang X, Kaeser-Woo YJ, Südhof TC, Malenka RC (2012), Postsynaptic complexin controls AMPA receptor exocytosis during LTP. Neuron 73: 260–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki Y, Lin DT, Huganir RL (2010), Plasma membrane insertion of the AMPA receptor GluA2 subunit is regulated by NSF binding and Q/R editing of the ion pore. Proc Natl Acad Sci USA 107:11080–11085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt KL, Zhang Y, Jurado S, Malenka RC, Südhof TC, Chen L (2015), Retinoic Acid and LTP Recruit Postsynaptic AMPA Receptors Using Distinct SNARE-Dependent Mechanisms. Neuron 86: 442–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascaño M, Richmond A, Borden P, Kuruvilla R (2009), Axonal targeting of Trk receptors via transcytosis regulates sensitivity to neurotrophin responses. J Neurosci 29: 11674–11685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, García-Arrarás JE, Elferink LA, Peterson K, Fleming AM, Hazuka CD, Scheller RH (1993), The syntaxin family of vesicular transport receptors. Cell 74:863–873 [DOI] [PubMed] [Google Scholar]
- Bin NR, Ma K, Harada H, Tien CW, Bergin F, Sugita K, et al. , (2018) Crucial Role of Postsynaptic Syntaxin 4 in Mediating Basal Neurotransmission and Synaptic Plasticity in Hippocampal CA1 Neurons. Cell Rep 23: 2955–2966. [DOI] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA (2003), AMPA receptor trafficking at excitatory synapses. Neuron 40: 361–379 [DOI] [PubMed] [Google Scholar]
- Brunger AT, Weninger K, Bowen M, Chu S (2009), Single-molecule studies of the neuronal SNARE fusion machinery. Annu Rev Biochem 78: 903–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H, Reim K, Varoqueaux F, Tapechum S, Hill K, Sørensen JB, Brose N, Chow RH (2008), Complexin II plays a positive role in Ca2+-triggered exocytosis by facilitating vesicle priming. Proc Natl Acad Sci USA 105: 19538–19543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Few AP (2008), Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901. [DOI] [PubMed] [Google Scholar]
- Chen D, Minger SL, Honer WG, Whiteheart SW. (1999), Organization of the secretory machinery in the rodent brain: distribution of the t-SNAREs, SNAP-25 and SNAP-23. Brain Res 831:11–24 [DOI] [PubMed] [Google Scholar]
- Clapp WC, Hamm JP, Kirk IJ, Teyler TJ (2012), Translating long-term potentiation from animals to humans: a novel method for noninvasive assessment of cortical plasticity. Biol. Psychiatry 71: 496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H (1983), Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol 334: 33–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condliffe SB, Corradini I, Pozzi D,Verderio C, Matteoli M (2010), Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J.Biol.Chem 285: 24968–24976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Bassani S, Brown TC, Lisé MF, Backos DS, El-Husseini A, Passafaro M, Esteban JA (2008), Motor protein-dependent transport of AMPA receptors into spines during long-term potentiation. Nat. Neurosci 1: 457–466 [DOI] [PubMed] [Google Scholar]
- Ehlers MD (2012), Hijacking Hebb: noninvasive methods to probe plasticity in psychiatric disease. Biol. Psychiatry 71: 484–486 [DOI] [PubMed] [Google Scholar]
- Elferink LA, Trimble WS, Scheller RH (1989) Two vesicle-associated membrane protein genes are differentially expressed in the rat central nervous system. J Biol Chem 264:11061–11064. [PubMed] [Google Scholar]
- Elfving B, Bonefeld BE, Rosenberg R, Wegener G (2008), Differential expression of synaptic vesicle proteins after repeated electroconvulsive seizures in rat frontal cortex and hippocampus. Synapse 62:662–670 [DOI] [PubMed] [Google Scholar]
- Ernst JA, Brunger AT (2003), High resolution structure, stability, and synaptotagmin binding of a truncated neuronal SNARE complex. J Biol Chem 278 :8630–8636 [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC (2001), Synaptotagmin I functions as a calcium regulator of release probability. Nature 410: 41–49 [DOI] [PubMed] [Google Scholar]
- Fong DK, Rao A, Crump FT, Craig AM (2002), Rapid synaptic remodeling by protein kinase C: reciprocal translocation of NMDA receptors and calcium/calmodulin-dependent kinase II. J Neurosci 22: 2153–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati G, Morini R, Corradini I, Antonucci F, Trepte P, Edry E ,et al. (2015), Reduced SNAP-25 increases PSD-95 mobility and impairs spine morphogenesis. Cell Death Differ 22: 1425–1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaisano HY, Ghai M, Malkus PN, Sheu L, Bouquillon A, Bennett MK, et al. (1996), Distinct cellular locations of the syntaxin family of proteins in rat pancreatic acinar cells. Mol. Biol. Cell 7: 2019–2027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Chiu SL, Liu B, Wu PH, Delannoy M, Lin DT, Wirtz D, Huganir RL (2016), Differential vesicular sorting of AMPA and GABAA receptors. Proc Natl Acad Sci U S A 113: E922–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Huganir RL (2016), Identification of the SNARE complex mediating the exocytosis of NMDA receptors. Proc Natl Acad Sci U S A 43:12280–12285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Zhang YV, Piccioli ZD, Perrimon N, Littleton JT (2016), The postsynaptic t-SNARE Syntaxin 4 controls traffic of Neuroligin 1 and Synaptotagmin 4 to regulate retrograde signaling. Elife 5: pii: e13881 10.7554/eLife.13881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R (2000), Driving AMPA receptors into synapses by LTP and CaMKII: Requirement for GluR1 and PDZ domain interaction. Science 287: 2262–2267 [DOI] [PubMed] [Google Scholar]
- Henley JM, Barker EA, Glebov OO (2011), Routes, destinations and delays: recent advances in AMPA receptor trafficking. Trends Neurosci 34: 258–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohenstein AC, Roche PA (2001), SNAP-29 is a promiscuous syntaxin-binding SNARE. Biochem Biophys Res Commun 285: 167–171 [DOI] [PubMed] [Google Scholar]
- Holderith N, Lorincz A, Katona G, Rózsa B, Kulik A, Watanabe M, et al. (2012), Release probability of hippocampal glutamatergic terminals scales with the size of the active zone. Nat.Neurosci 15: 988–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt M, Varoqueaux F, Wiederhold K, Takamori S, Urlaub H, Fasshauer D, Jahn R (2006), Identification of SNAP-47, a novel Qbc-SNARE with ubiquitous expression. J Biol Chem 281: 17076–17083 [DOI] [PubMed] [Google Scholar]
- Huang G-Z, Ujihara H, Takahashi S, Kaba H, Yagi T, Inoue S (2000), Involvement of complexin II in synaptic plasticity in the CA1 region of the hippocampus: the use of complexin II-lacking mice. Jpn J Pharmacol 84: 179–187 [DOI] [PubMed] [Google Scholar]
- Hussain S, Davanger S (2015), Postsynaptic VAMP/Synaptobrevin Facilitates Differential Vesicle Trafficking of GluA1 and GluA2 AMPA Receptor Subunits. PLoS One 10:e0140868 doi: 10.1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Ringsevjen H, Egbenya DL, Skjervold TL, Davanger S (2016), SNARE Protein Syntaxin-1 Colocalizes Closely with NMDA Receptor Subunit NR2B in Postsynaptic Spines in the Hippocampus. Front Mol Neurosci 9:10 10.3389/fnmol.2016.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman SL, Turecek J, Belinsky JE, Regehr WG (2016), The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature 529: 88–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Scheller RH (2006), SNAREs–engines for membrane fusion. Nat Rev Mol Cell Biol 9: 631–643 [DOI] [PubMed] [Google Scholar]
- Jahn R, Südhof TC (1999), Membrane fusion and exocytosis. Annu Rev Biochem 68: 863–911 [DOI] [PubMed] [Google Scholar]
- Jeyifous O, Waites CL, Specht CG, Fujisawa S, Schubert M, Lin EI, Marshall J, Aoki C, de et al. (2009), SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat Neurosci 12: 1011–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan BA, Fernholz BD, Boussac M, Xu C, Grigorean G, Ziff EB, Neubert TA (2004), Identification and verification of novel rodent postsynaptic density proteins. Mol Cell Proteomics 3: 857–871 [DOI] [PubMed] [Google Scholar]
- Jurado S, Goswami D, Zhang Y, Molina AJ, Südhof TC, Malenka RC (2013), LTP requires a unique postsynaptic SNARE fusion machinery. Neuron 77:542–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD (2011), Mechanisms and function of dendritic exocytosis. Neuron 69: 856–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MJ, Davison IG, Robinson CG, Ehlers MD (2010), Syntaxin-4 defines a domain for activity-dependent exocytosis in dendritic spines. Cell 14: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti K, Lorincz A, Nusser Z (2012), Unique somato-dendritic distribution pattern of Kv4.2 channels on hippocampal CA1 pyramidal cells. Eur.J. Neurosci 35: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh S, Yamamoto A, Inoue A, Inoue Y, Akagawa K, Kawamura Y, et al. (1993), Immunoelectron microscopic localization of the HPC-1 antigen in rat cerebellum. J. Neurocytol 22: 995–1005. 10.1007/bf01218356 [DOI] [PubMed] [Google Scholar]
- Kuster A, Nola S, Dingli F, Vacca B, Gauchy C, Beaujouan JC, Nunez M, Moncion T, Loew D, Formstecher E, Galli T, Proux-Gillardeaux V (2015), The Q-soluble N-Ethylmaleimide-sensitive Factor Attachment Protein Receptor (Q-SNARE) SNAP-47 Regulates Trafficking of Selected Vesicle-associated Membrane Proteins (VAMPs). J Biol Chem 47: 28056–28069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau CG, Takayasu Y, Rodenas-Ruano A, Paternain AV, Lerma J, Bennett MV, Zukin RS (2010), SNAP-25 is a target of protein kinaseC phosphorylation critical to NMDA receptor trafficking. J Neurosci 30: 242–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Bruns D, Wenzel D, Riedel D, Holroyd P, Thiele C, Jahn R (2001), SNAREs are concentrated in cholesterol-dependent clusters that define docking and fusion sites for exocytosis. EMBO J 20: 2202–2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang T, Jahn R (2008), Core proteins of the secretory machinery. Handb Exp Pharmacol 184:107–127 [DOI] [PubMed] [Google Scholar]
- Liu XB, Murray KD, Jones EG (2004), Switching of NMDA receptor 2A and 2B subunits at thalamic and cortical synapses during early postnatal development”. J. Neurosci 40: 8885–8895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lledó PM, Zhang X, Südhof TC, Malenka RC, Nicoll RA (1998), Postsynaptic membrane fusion and long-term potentiation. Science 279: 399–403 [DOI] [PubMed] [Google Scholar]
- Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T (2006), Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell 17: 977–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Helton TD, Blanpied TA, Rácz B, Newpher TM, Weinberg RJ, Ehlers MD (2007), Postsynaptic positioning of endocytic zones and AMPA receptor cycling by physical coupling of dynamin-3 to Homer. Neuron 55: 874–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT (2001), Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron 29: 243–254 [DOI] [PubMed] [Google Scholar]
- Lüscher C, Xia H, Beattie EC, Carroll RC, von Zastrow M, Malenka RC, Nicoll RA (1999), Role of AMPA receptor cycling in synaptic transmission and plasticity. Neuron 24: 649–658 [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF (2004), LTP and LTD: an embarrassment of riches. Neuron 44: 5–21 [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC (2002), AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci 25, 103–126 [DOI] [PubMed] [Google Scholar]
- McMahon HT, Missler M, Li C, Südhof TC (1995), Complexins: cytosolic proteins that regulate SNAP receptor function. Cell 83: 111–119 [DOI] [PubMed] [Google Scholar]
- Morelli E, Ginefra P, Mastrodonato V, Beznoussenko GV, Rusten TE, Bilder D, Stenmark H, Mironov AA, Vaccari T (2014), Multiple functions of the SNARE protein Snap29 in autophagy, endocytic, and exocytic trafficking during epithelial formation in Drosophila. Autophagy 10: 2251–2268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster-Wandowski A, Heilmann H, Bolduan F, Trimbuch T, Yanagawa Y, Vida I, (2017), Distinct Localization of SNAP47 Protein in GABAergic and Glutamatergic Neurons in the Mouse and the Rat Hippocampus. Front Neuroanat 11:56 10.3389/fnana.2017.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi H, Yasuda R (2012), Postsynaptic signaling during plasticity of dendritic spines. Trends Neurosci 35:135–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves G, Cooke SF, Bliss TV (2008), Synaptic plasticity, memory and the hippocampus: a neural network approach to causality. Nat. Rev. Neurosci 9: 65–75 [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD (2008), Glutamate receptor dynamics in dendritic microdomains. Neuron 58: 472–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara-Imaizumi M, Nishiwaki C, Kikuta T, Kumakura K, Nakamichi Y, Nagamatsu S (2004), Site of docking and fusion of insulin secretory granules in live MIN6 beta cells analyzed by TAT-conjugated anti-syntaxin 1 antibody and total internal reflection fluorescence microscopy. J Biol Chem 279: 8403–8408 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Schoch S, Südhof TC (1999), EHSH1/intersectin, a protein that contains EH and SH3 domains and binds to dynamin and SNAP-25. A protein connection between exocytosis and endocytosis? J. Biol. Chem 274: 18446–18454 [DOI] [PubMed] [Google Scholar]
- Opazo P, Choquet D (2011), A three-step model for the synaptic recruitment of AMPA receptors. Mol. Cell. Neurosci 46: 1–8 [DOI] [PubMed] [Google Scholar]
- Pabst S, Hazzard JW, Antonin W, Südhof TC, Jahn R, Rizo J, Fasshauer D (2000), Selective interaction of complexin with the neuronal SNARE complex. Determination of the binding regions. J Biol Chem 275: 19808–19818 [DOI] [PubMed] [Google Scholar]
- Pantano S, Montecucco C (2014), The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell. Mol. Life Sci 71:793–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson MA, Szatmari EM, Yasuda R (2010), AMPA receptors are exocytosed in stimulated spines and adjacent dendrites in a Ras-ERK-dependent manner during long-term potentiation. Proc. Natl. Acad. Sci. USA 107: 15951–15956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini EM, Lu J, Cognet L, Lounis B, Ehlers MD, Choquet D.(2009), Endocytic trafficking and recycling maintain a pool of mobile surface AMPA receptors required for synaptic potentiation. Neuron 63: 92–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, Lauffer B, Temkin P, Vistein R, Carlton P, Thorn K, Taunton J, Weiner OD, Parton RG, von Zastrow M (2010), Sequence-dependent sorting of recycling proteins by actin-stabilized endosomal microdomains. Cell 143: 761–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raptis A, Torrejon-Escribano B, Gomez de Aranda I, Blasi J (2005) Distribution of synaptobrevin/VAMP 1 and 2 in rat brain. J Chem Neuroanat 30:201–211. [DOI] [PubMed] [Google Scholar]
- Ravichandran V, Chawla A, Roche PA (1996), Identification of a novel syntaxin- and synaptobrevin/VAMP-binding protein, SNAP-23, expressed in non-neuronal tissues. J Biol Chem 271: 13300–13303 [DOI] [PubMed] [Google Scholar]
- Rizo J, Rosenmund C (2008), Synaptic vesicle fusion. Nat Struct Mol Biol 15: 665–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotem-Yehudar R, Galperin E, Horowitz M (2001), Association of insulin-like growth factor 1 receptor with EHD1 and SNAP29. J Biol Chem 276: 33054–33060 [DOI] [PubMed] [Google Scholar]
- Sadoul K, Berger A, Niemann H, Weller U, Roche PA, Klip A et al. (1997), SNAP-23 is not cleaved by botulinum neurotoxin E and can replace SNAP-25 in the process of insulin secretion. J. Biol. Chem 272: 33023–33027 [DOI] [PubMed] [Google Scholar]
- Saito T, Guan F, Papolos DF, Rajouria N, Fann CS, Lachman HM (2001), Polymorphism in SNAP29 gene promoter region associated with schizophrenia. Mol Psychiatry 6: 193–201 [DOI] [PubMed] [Google Scholar]
- Sampo B, Kaech S, Kunz S, Banker G (2003), Two distinct mechanisms target membrane proteins to the axonal surface. Neuron 37: 611–624 [DOI] [PubMed] [Google Scholar]
- Schardt A, Brinkmann BG, Mitkovski M, Sereda MW, Werner HB, Nave KA (2009), The SNARE protein SNAP-29 interacts with the GTPase Rab3A: Implications for membrane trafficking in myelinating glia. J Neurosci Res 87: 3465–3479 [DOI] [PubMed] [Google Scholar]
- Schonn JS, Maximov A, Lao Y, Südhof TC, Sørensen JB (2008), Synaptotagmin-1 and −7 are functionally overlapping Ca2+ sensors for exocytosis in adrenal chromaffin cells. Proc Natl Acad Sci USA 105: 3998–4003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak S, Paternain AV, Aller MI, Picó E, Rivera R, Lerma J (2009), A role for SNAP25 in internalization of kainite receptors and synaptic plasticity. Neuron 63: 357–371 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL (2007), The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol 23: 613–643 [DOI] [PubMed] [Google Scholar]
- Shimojo M, Courchet J, Pieraut S, Torabi-Rander N, Sando R 3rd, Polleux F, Maximov A (2015), SNAREs Controlling Vesicular Release of BDNF and Development of Callosal Axons. Cell Rep 7: 1054–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber JJ, Willig KI, Kutzner C, Gerding-Reimers C, Harke B, Donnert G, Rammner B, Eggeling C, Hell SW, Grubmuller H, et al. (2007), Anatomy and dynamics of a supramolecular membrane protein cluster. Science 317: 1072–1076 [DOI] [PubMed] [Google Scholar]
- Sørensen JB, Nagy G,Varoqueaux F, Nehring RB, Brose N, Wilson MC, et al. (2003), Differential control of the releasable vesicle pools by SNAP-25 splice variants and SNAP-23. Cell 114: 75–86 [DOI] [PubMed] [Google Scholar]
- Sprengel R, Suchanek B, Amico C, Brusa R, Burnashev N, Rozov A, et al. (1998), Importance of the intracellular domain of NR2 subunits for NMDA receptor function in vivo. Cell 92: 279–289 [DOI] [PubMed] [Google Scholar]
- Steegmaier M, Yang B, Yoo JS, Huang B, Shen M, Yu S, Luo Y, Scheller RH (1998), Three novel proteins of the syntaxin SNAP-25 family. J Biol Chem 273: 34171–34179 [DOI] [PubMed] [Google Scholar]
- Su Q, Mochida S, Tian JH, Mehta R, Sheng ZH (2001), SNAP-29: a general SNARE protein that inhibits SNARE disassembly and is implicated in synaptic transmission. Proc Natl Acad Sci U S A 98:14038–14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2008), Neuroligins and neurexins link synaptic function to cognitive disease. Nature 455:903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2008), Neurotransmitter release. Handb Exp Pharmacol 184:1–21 [DOI] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE (2009), Membrane fusion: grappling with SNARE and SM proteins. Science 323: 474–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA (2010), A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat Neurosci 13: 338–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT (1998), Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature 395: 347–353 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ujihara H, Huang G-Z, Yagyu KI, Sanbo M, Kaba H, Yagi T (1999), Reduced hippocampal LTP in mice lacking a presynaptic protein: complexin II. Eur J Neurosci 11, 2359–2366 [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, et al. , (2006), Molecular anatomy of a trafficking organelle cell, Cell 127: 831–846 [DOI] [PubMed] [Google Scholar]
- Tao-Cheng JH, Pham A, Yang Y, Winters CA, Gallant PE, Reese TS (2015), Syntaxin 4 is concentrated on plasma membrane of astrocytes. Neuroscience 286: 264–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M (2011), SNX27 mediates retromer tubule entry and endosometo-plasma membrane trafficking of signalling receptors. Nat Cell Biol 13: 715–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni R, Repetto D, Morini R, Elia C, Gardoni F, Di Luca M, Turco E, Defilippi P, Matteoli M (2013), SNAP-25 regulates spine formation through postsynaptic binding to p140Cap. Nat Commun 4: 2136. [DOI] [PubMed] [Google Scholar]
- Trimble WS, Gray TS, Elferink LA, Wilson MC, Scheller RH (1990), Distinct patterns of expression of two VAMP genes within the rat brain. JNeurosci 10:1380–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidyanathan VV, Puri N, Roche PA. (2001), The last exon of SNAP-23 regulates granule exocytosis from mast cells. J. Biol. Chem 276: 25101–25106 [DOI] [PubMed] [Google Scholar]
- Verderio C, Pozzi D, Pravettoni E, Inverardi F, Schenk U, Coco S, et al. (2004), SNAP-25 modulation of calcium dynamics underlies differences in GABAergic and glutamatergic responsiveness to depolarization. Neuron 41: 599–610 [DOI] [PubMed] [Google Scholar]
- Wang Z, Edwards JG, Riley N, Provance DW Jr, Karcher R, Li XD, Davison IG, Ikebe M, Mercer JA, Kauer JA, Ehlers MD (2008), Myosin Vb mobilizes recycling endosomes and AMPA receptors for postsynaptic plasticity. Cell 135: 535–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Bendito G, Molnar Z, et al. (2002), Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat Neurosci 5: 19–26 [DOI] [PubMed] [Google Scholar]
- Wong SH, Xu Y, Zhang T, Griffiths G, Lowe SL, Subramaniam VN, Seow KT, Hong W (1999), Mol. Biol. Cell 10: 119–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Bacaj T, Morishita W, Goswami D, Arendt KL, Xu W, Chen L, Malenka RC, Südhof TC (2017), Postsynaptic synaptotagmins mediate AMPA receptor exocytosis during LTP. Nature 544: 316–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Mashimo T, Südhof TC (2007), Synaptotagmin-1, −2, and −9: Ca(2+) sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron 54: 567–581 [DOI] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q (2008), Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc. Natl. Acad. Sci. USA 105: 11388–11393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap CC, Wisco D, Kujala P, Lasiecka ZM, Cannon JT, Chang MC, Hirling H, Klumperman J, Winckler B (2008), The somatodendritic endosomal regulator NEEP21 facilitates axonal targeting of L1/NgCAM. J Cell Biol 180: 827–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin DM, Chen YJ, Sathyamurthy A, Xiong WC, Mei L (2012), Synaptic dysfunction in schizophrenia. Adv Exp Med Biol 970:493–516 [DOI] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M (2007), Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J. Neurosci 27: 11112–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco D, Anderson ED, Chang MC, Norden C, Boiko T, Fölsch H, Winckler B (2003), Uncovering multiple axonal targeting pathways in hippocampal neurons. J Cell Biol 162: 1317–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wang D, Sun T, Xu J, Chiang HC, Shin W, et al. (2013), The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J. Neurosci 33: 9169–9175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Baudry M (2006), Developmental changes in NMDA neurotoxicity reflect developmental changes in subunit composition of NMDA receptors. J. Neurosci 26: 2956–2963 [DOI] [PMC free article] [PubMed] [Google Scholar]


