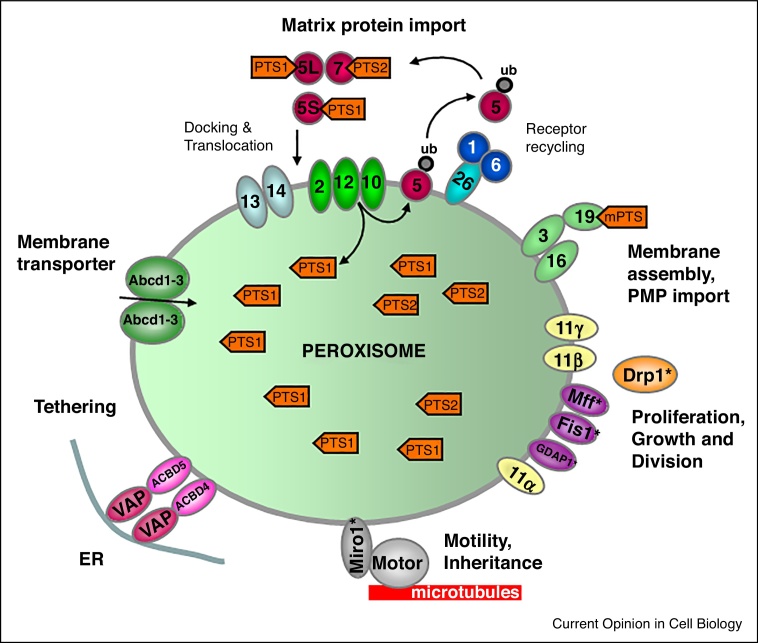
Figure 1.
Schematic overview of the molecular machineries involved in the biogenesis of mammalian peroxisomes. Matrix protein import: After synthesis on free ribosomes, cargo proteins containing the peroxisomal targeting signals PTS1 or PTS2 bind to the corresponding cytosolic receptors Pex5 or Pex7 and form receptor-cargo complexes. The Pex7–cargo complex requires Pex5L, the long isoform of Pex5, for import. Import is achieved by a complex set of integral or peripheral PMPs that form the matrix protein import machinery, which mediates docking of the cargo-bound import receptor at the peroxisomal membrane, cargo translocation into the matrix of the organelle by a dynamic translocon, and export of the receptor back to the cytosol. Recycling of the receptor involves its ubiquitination (ub) and extraction from the membrane by an AAA-ATPase complex (Pex1, Pex6). Membrane assembly and insertion of PMPs (containing an mPTS) depends on Pex19, Pex3 and Pex16. Pex19 functions as a cycling receptor/chaperone, which binds the PMPs in the cytosol and interacts with Pex3 at the peroxisomal membrane. Proliferation, growth and division: Pex11α, Pex11β and Pex11γ are involved in the regulation of peroxisome size and number. Pex11β remodels the peroxisomal membrane, and interacts with the membrane adaptors Mff and Fis1, which recruit the dynamin-like fission GTPase Drp1 to peroxisomes, which is activated by Pex11β. Motility and Inheritance: Miro1 serves as membrane adaptor for the microtubule-dependent motor proteins kinesin and dynein [19•, 38••, 64••]. Tethering: ACBD5 and ACBD4 interact with ER-resident VAPA/B to mediate peroxisome-ER contacts. Membrane transporter: only the ABC transporter proteins involved in fatty acid uptake are shown. Proteins with a dual localisation to both peroxisomes and mitochondria are marked with an asterisk.