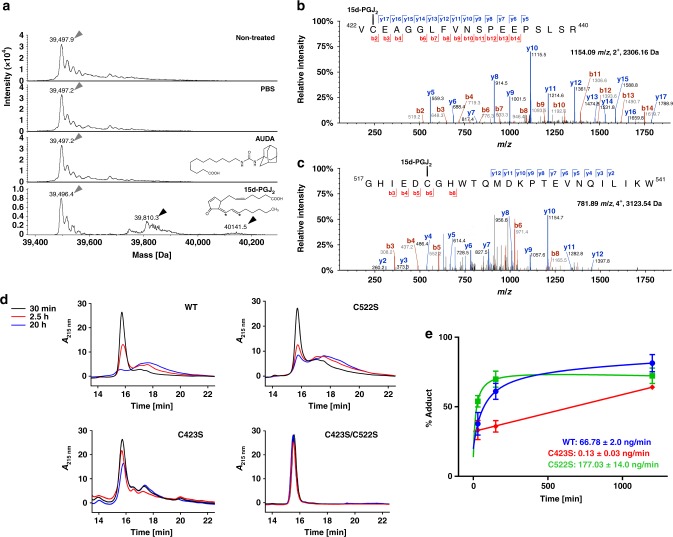
Fig. 1.
Analysis of the covalent interaction between hsEH CTD and 15d-PGJ2. a ESI-MS experiments. Grey and black arrows indicate the free and covalently modified hsEH CTD, respectively. The electrophilic carbon atoms of 15d-PGJ2 are indicated by asterisks. b LC-MS/MS evidence of C423 modification. A peptide with m/z 1154.092+ was assigned through the identification of ions b2-b4, b6-b14 and y5-y17. The direct assignment of the modification on both b2-ions and y17-ions was strong evidence of modification of C423. c LC-MS/MS evidence of C522 modification. The peptide exhibited a m/z of 781.894+. Its sequence was assigned through the detection of b3-b6, b8 and y2-y12 ions. The b6 ion modification allowed the direct identification of C522 adduction. d HPLC separation of the covalent complexes. The traces were collected at three different time points, upon incubation of hsEH CTD and 15d-PGJ2. For clarity, the chromatograms of the free proteins are reported in Supplementary Fig. 1. e 15d-PGJ2 rate of adduction. Rates were calculated from deconvolution of the HLPC traces shown in d, as described in the Methods section. The mutant C423S showed a considerably lower rate of adduction to C522, suggesting that the kinetics of modification of the two residues are different. Data presented as average ± SEM of n = 4 WT, n = 3 C522, and n = 3 C423S. (Source data available in Supplementary Data 1)