Fig. 3.
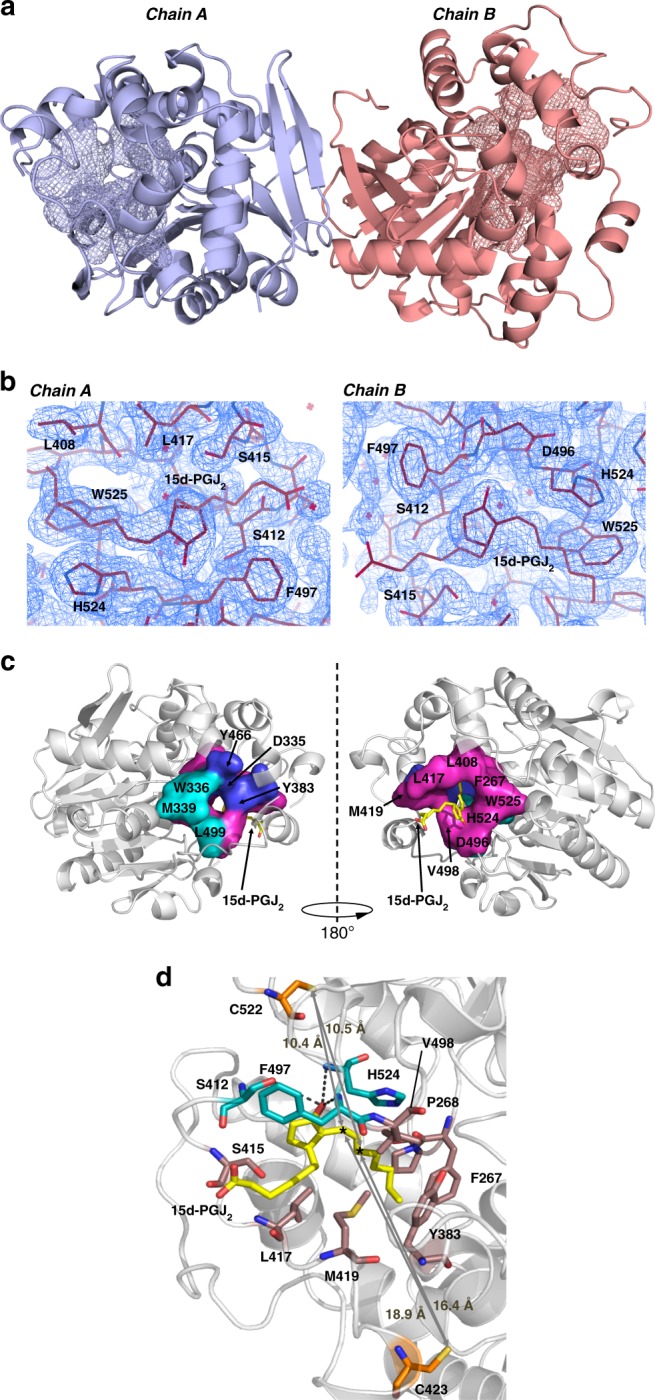
X-ray crystallographic structure of the 15d-PGJ2-hsEH CTD complex. a Crystallographic symmetry. The crystallographic homodimer is represented as a cartoon model (chain A and B in light blue and salmon red respectively), with the ‘L’-shaped catalytic site in mesh. b Electron density of the 15d-PGJ2 bound to hsEH CTD. The 2Fc-Fo map was contoured at 1 sigma. c 15d-PGJ2 binding site. The ‘L-shaped’ catalytic site of hsEH CTD is represented with a three-colours code: the F267 Pocket in magenta; the W336 Niche in cyan; the ‘L’ vertex formed by the catalytic D335 and the epoxide positioners Y363 and Y466 are represented in blue. d Overall 15d-PGJ2 interaction with hsEH CTD. The amino acids involved in van der Waals interaction are depicted in brown, while those forming hydrogen bonds with 15d-PGJ2 are in dark cyan. C423 and C522 are highlighted in orange, and the reactive electrophilic C13 and C15 atoms of the ligand are indicated by asterisks
