Fig. 2.
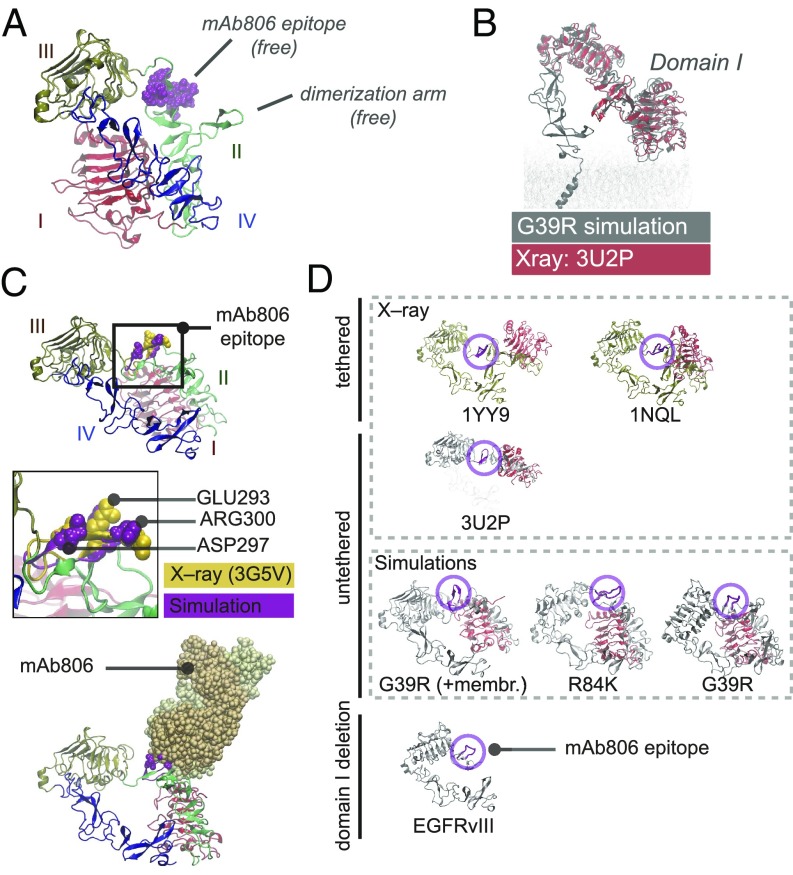
Twisted untethered intermediates suggest structural convergence of N-TR1 reposition and its deletion in EGFRvIII. (A) MD twisted conformers display intermediate conformations characterized by N-TR1 rotation that renders the 806-epitope and the dimerization arm free. (B) The tethered-like arrangement of domains I to III in MD intermediates overlaps with that observed for tether-truncated ECDs (e.g., 3U2P in red). (C) In twisted intermediates, the MD 806-epitope (purple) has a convex surface with key residues for mAb806 binding protruding to the solvent and aligning with X-ray structures of antigen–antibody complexes (3G5V, yellow) (Top). N-TR1 rotation removes stereochemical overlap for docking onto 3G5V, rendering stable MD complexes with mAb806 (Bottom). (D) Domain I (red) sterically occludes 806-epitope (magenta) in tethered conformations; the hindrance is greater in planar configurations (1YY9) than in low-twist tethered structures (1NQL) and minimal in the truncated ECD (3U2P). Both deletion (EGFRvIII) and rotation of N-TR1 (G39R/R84K intermediates) removes domain I from the tethered position that hinders the epitope. In I-II mutations, N-TR1 reposition allows receptor–receptor docking via dimerization arms (SI Appendix, Fig. S4B).