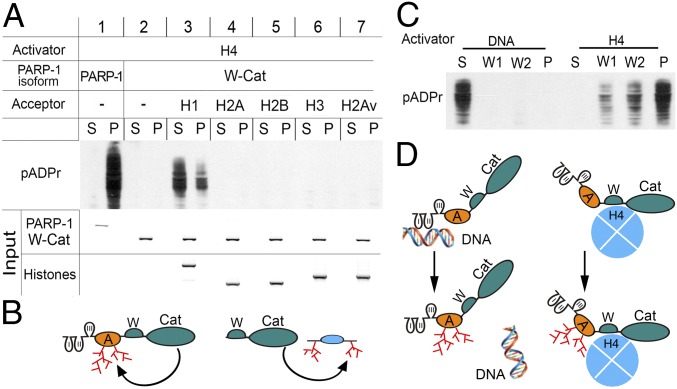
Fig. 4.
Regulation and targeting of enzymatic activity of PARP-1 by histones and DNA. (A–B) PARP-1 modifies either itself via the A domain (A) or the linker histone H1 in vitro. (A) Binding-activation assay: full-length PARP-1 and deletional isoform W-Cat were separately incubated with H4-bound sepharose beads, washed, and mixed with NAD and other histones (H1, H2A, H2B, H3, and H2Av) to induce pADPr production. The supernatant (S) was removed and the pellet (P) was washed. The absence of the A domain in the W-Cat isoform precludes automodification of PARP-1. Histones were added as potential substrates for the poly(ADP ribosyl)ation assay. Accumulation of pADPr was detected on Western blot using an anti-pADPr antibody (10H). Input: the Coomassie Brilliant Blue stained PAGE gels show the amounts of PARP-1, W-Cat, and histones loaded in each reaction. (B) Diagram illustrating the activity of full-length PARP-1 and PARP-1 deletional isoforms via the A domain and modification of H1. (C–D) Upon activation and automodification, PARP-1 loses interaction with DNA but remains bound to histone H4. (C) Interactive-activity assay of PARP-1 with sepharose beads coupled to either nicked DNA or H4. PARP-1 was incubated separately with either nicked DNA- or H4-coupled beads, washed, and mixed with NAD to trigger pADPr production. The solution was removed and the pellet was washed twice (W1, W2). The distribution of pADPr between fractions was measured after PAGE on a Western blot using an anti-pADPr antibody. (D) Diagram illustrating that automodified PARP-1 dissociates from DNA but remains bound to H4.