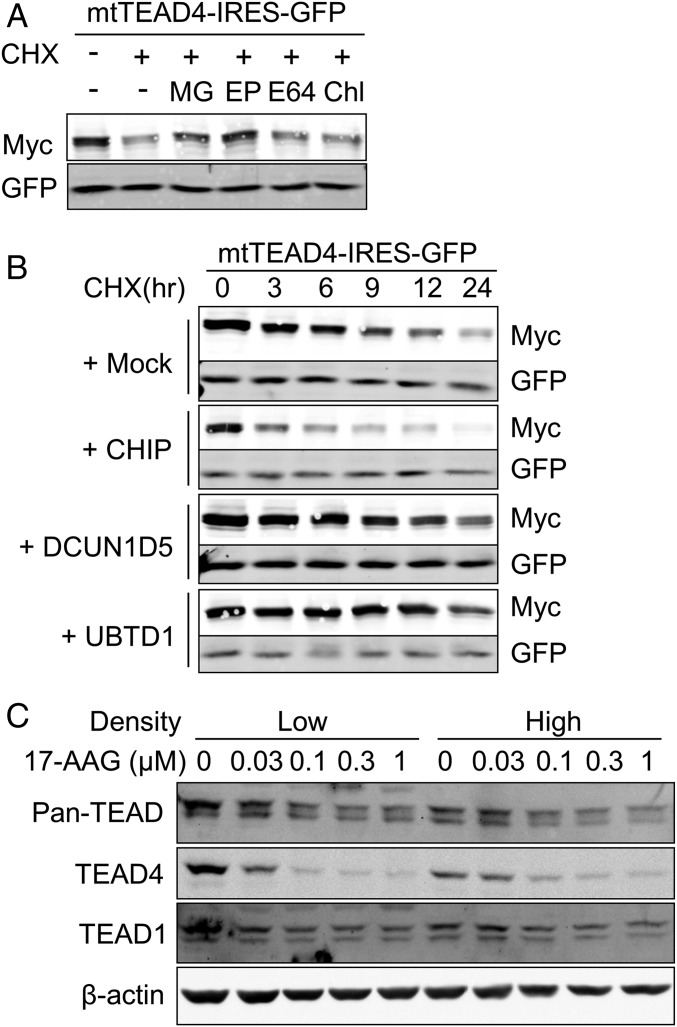
Fig. 5.
E3 ubiquitin ligase CHIP promotes the degradation of unpalmitoylated TEAD4. (A) Inhibition of palmitoylation-deficient TEAD4 degradation by proteasome inhibitors. 293A cells transfected with palmitoylation-deficient TEAD4-IRES-GFP (mtTEAD4-IRES-GFP) were treated with 10 μM CHX in the presence of MG (10 μM MG-132, proteasome inhibitor), EP (1 μM Epoxomicin, proteasome inhibitor), E64 (10 μM E-64, cysteine proteases inhibitor), or Chl (2.5 μM Chloroquine, autophagy inhibitor) for 9 h. The expression levels of mutant-TEAD4 and GFP were analyzed by Western blot. (B) Promotion of the degradation of unpalmitoylated TEAD4 by E3 ubiquitin ligase CHIP. 293A cells transfected with mtTEAD4-IRES-GFP and indicated E3-ubiquitin ligases. After 24 h of transfection, cells were treated with 10 μM CHX for the indicated time, followed by Western blot analysis. (C) Degradation of TEAD stimulated by the Hsp90 inhibitor 17-AAG. MCF-10A cells at low or high densities were treated with 17-AAG for 24 h at the indicated concentrations. MCF-10A cells at low or high densities were treated for 24 h with 17-AAG at the indicated concentrations. TEAD protein level was observed by Western blot.