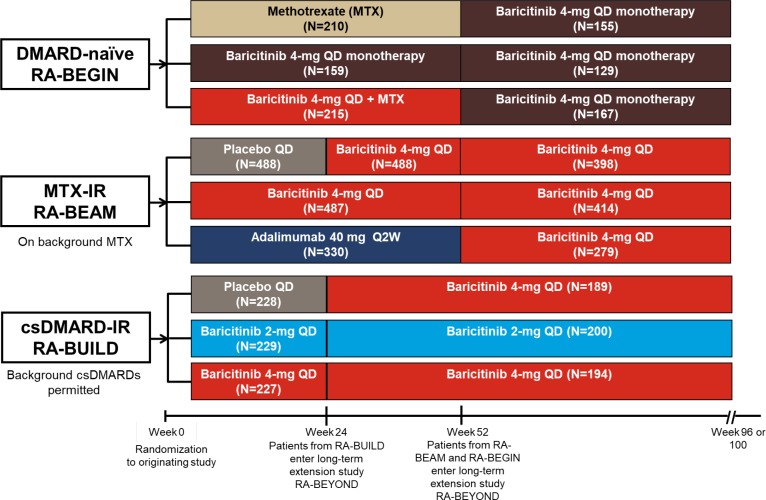
Figure 1.
Design for the long-term extension study RA-BEYOND from randomisation in the originating studies. Data are up to 2 years on treatment, corresponding to 100 weeks in RA-BEGIN and RA-BEAM or 96 weeks in RA-BUILD due to the different duration and visit structures of the originating studies. Patients from RA-BEGIN switched to baricitinib 4 mg at entry to RA-BEYOND at week 52; during RA-BEYOND, MTX or other csDMARDs could be prescribed at any time point at the discretion of the investigators. Patients from RA-BEAM originally randomised to placebo were switched to baricitinib 4 mg at rescue or week 24; patients randomised to adalimumab were switched to baricitinib 4 mg at rescue or entry to RA-BEYOND at week 52. Patients in RA-BUILD initially randomised to placebo switched to baricitinib 4 mg at rescue or week 24 at entry to RA-BEYOND. Patients who remained on baricitinib 2 mg in RA-BUILD continued on 2 mg in RA-BEYOND; those rescued from baricitinib 2 mg to 4 mg in RA-BUILD received baricitinib 4 mg in RA-BEYOND. Background csDMARDs were permitted in RA-BUILD and RA-BEAM. csDMARD, conventional synthetic DMARD; DMARD, disease-modifying antirheumatic drug; IR, inadequate responder; MTX, methotrexate; N, number of patients randomised and treated; Q2W, once every 2 weeks; QD, once daily; RA, rheumatoid arthritis.