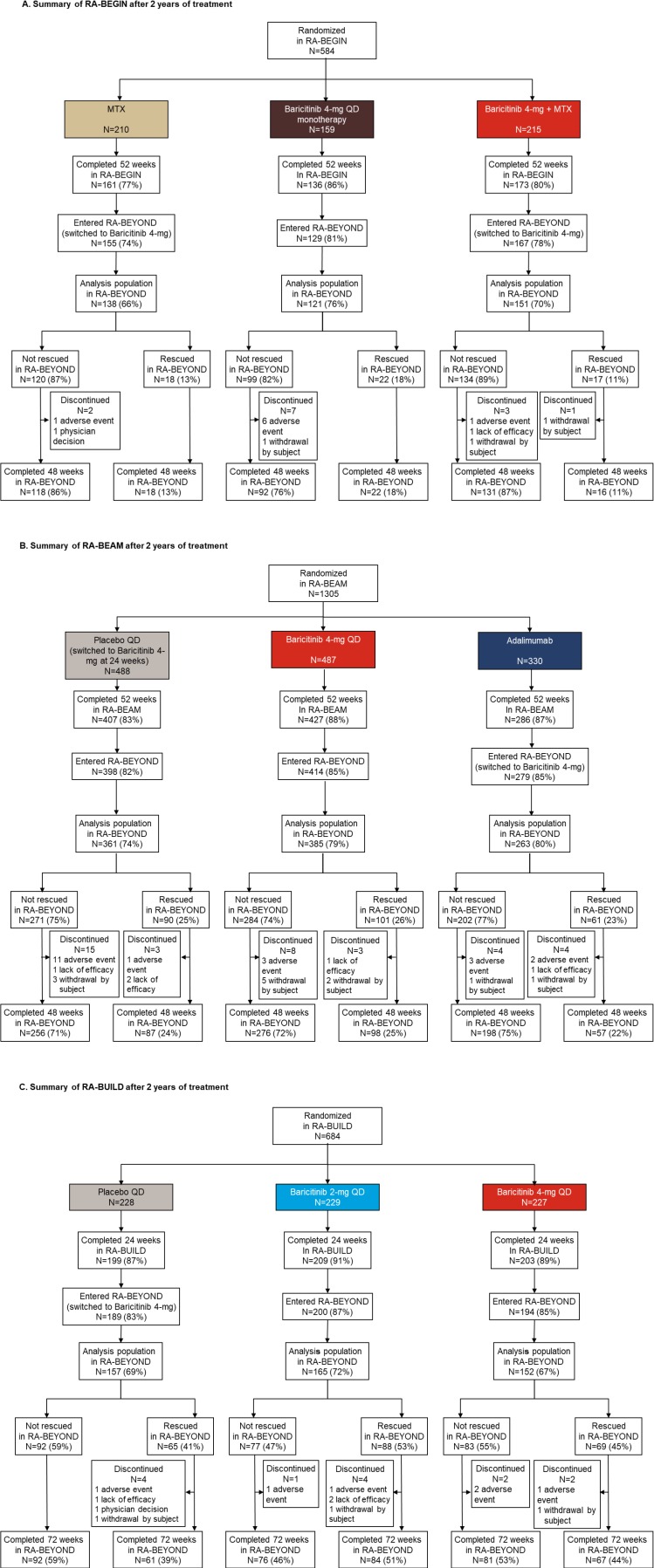
Figure 2.
Patient disposition after 2 years of treatment. Summary of the analysis population in RA-BEYOND for patients originally completing (A) RA-BEGIN, (B) RA-BEAM or (C) RA-BUILD. Disposition is shown for up to 2 years on treatment, corresponding to a total of 100 weeks in RA-BEGIN and RA-BEAM (52 weeks in the originating study plus 48 weeks in RA-BEYOND) and 96 weeks in RA-BUILD (24 weeks in originating study plus 72 weeks in RA-BEYOND) due to the different durations and visit structures of the originating studies. Patients on placebo, MTX or adalimumab in the originating studies switched to baricitinib 4 mg at rescue or at 24 or 52 weeks, as detailed in the Study design section. The analysis populations in each group were the patients with radiographic assessments at baseline of the originating study and at least one radiograph from RA-BEYOND after the 1-year radiograph. LTE, long-term extension; MTX, methotrexate; QD, once daily; RA, rheumatoid arthritis.