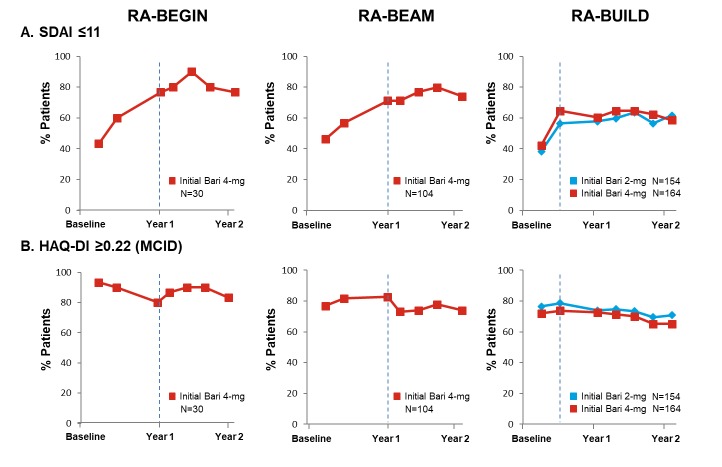
Figure 5.
Proportion of patients (NRI) on baricitinib achieving SDAI ≤11 and meeting or exceeding HAQ-DI MCID (≥0.22) up to year 2 by original randomisation groups. Baseline in the originating study was used in the calculation of response. Time points represent time from randomisation in originating study, with year 1 and year 2 corresponding respectively to week 52 and week 100 for RA-BEGIN and RA-BEAM and to week 48 and week 96 for RA-BUILD due to the different durations and visit structures of the originating studies. Time points between year 1 and year 2 are in 12-week intervals. Dotted lines indicate entry into the long-term extension study. Analyses exclude patients who were rescued in the originating studies. NRI was applied without regard to rescue status in RA-BEYOND. Data after patients stepped down to baricitinib 2 mg were imputed using the response rate of the patients who continued with baricitinib 4 mg. ‘Initial’ denotes initial randomised treatment group. Bari, baricitinib; HAQ-DI, Health Assessment Questionnaire–Disability Index; MCID, minimally clinically important difference; N, number of patients not rescued in the originating study, who continued on the same dose in RA-BEYOND and entered RA-BEYOND 96 weeks before the cut-off date; NRI, non-responder imputation; SDAI, Simplified Disease Activity Index; RA, rheumatoid arthritis.