Abstract
In neurons, Glycogen Synthase Kinase-3β (GSK-3β) has been shown to regulate various critical processes underlying structural and functional synaptic plasticity. Mouse models with neuron-selective expression or deletion of GSK-3β present behavioral and cognitive abnormalities, positioning this protein kinase as a key signaling molecule in normal brain functioning. Furthermore, mouse models with defective GSK-3β activity display distinct structural and behavioral abnormalities, which model some aspects of different neurological and neuropsychiatric disorders. Equalizing GSK-3β activity in these mouse models by genetic or pharmacological interventions is able to rescue some of these abnormalities. Thus, GSK-3β is a relevant therapeutic target for the treatment of many brain disorders. Here, we provide an overview of how GSK-3β is regulated in physiological synaptic plasticity and how aberrant GSK-3β activity contributes to the development of dysfunctional synaptic plasticity in neuropsychiatric and neurodegenerative disorders.
1. Neuronal Plasticity
Neural plasticity is an ability of the brain to adapt in response to normal developmental processes, experience, or injury. It covers such modifications in the brain structures as growth of new neurons, the formation of new networks, and change within existing networks, that is, changes in synaptic strengths, resulting in modifications in function and behavior.
2. Synaptic Plasticity
Reversible modification of synaptic strength underlies synaptic plasticity and is activity dependent. Synaptic strength can either be enhanced in a process of long-term potentiation (LTP) or depressed in long-term depression (LTD), and it affects both pre- and postsynaptic sides. LTP is triggered by the intense activation of the NMDA receptor producing a signaling cascade that causes the recruitment of AMPA receptors into the postsynaptic membrane, whereas LTD is triggered by weaker and prolonged activation of NMDA receptors leading to the removal of postsynaptic AMPA receptors [1]. Majority of the excitatory synapses are located on dendritic spines, and their growth following LTP and elimination following LTD are two opposite facts accompanying the bidirectional plasticity of excitatory transmission. Formation of new spines, as well as their morphological modifications in the adult brain, constitutes the structural bases of neuronal plasticity. The dynamic changes of dendritic spine morphology reflect changes in synaptic strength according to its use or disuse. It should be noted, however, that other forms of synaptic plasticity exist which add to the complexity of glutamatergic synapses [2].
On the other hand, inhibitory synaptic transmission driven by the interaction of GABA and ionotropic GABAA receptors constitutes a major form of inhibitory synaptic transmission. Loss of synaptic stability caused by improper excitatory/inhibitory balance and trafficking of synaptic receptors as well as abnormal density and morphology of dendritic spines may lead to the disruption of neuronal circuits resulting in neuropsychiatric disorders. The underlying mechanisms remain to be elucidated, but they depend essentially on kinase-dependent signaling pathways [3, 4].
3. Glycogen Synthase Kinase-3
Glycogen Synthase Kinase-3 (GSK-3) is a serine/threonine protein kinase that was first discovered for its role in glycogen synthesis [5]. Later on, extensive studies have implicated GSK-3 in the regulation of many critical cellular processes with over 40 different proteins identified as phosphorylation targets for GSK-3 [6].
GSK-3 exists as two isozymes, GSK-3 alpha (α) and GSK-3 beta (β), both of which are encoded by distinct genes [7]. They split from the common ancestor at the emergence of vertebrates, while birds lost GSK-3α in the evolution [8]. GSK-3α and β share 85% amino acid sequence similarity, including 98% sequence identity within their catalytic domains [7]. Despite their structural similarity, GSK-3α and GSK-3β are not functionally identical because the beta isozyme is indispensable in development [9, 10]. In mammals, both GSK-3 isozymes are ubiquitously expressed in all tissues [7], but they are most abundant in the adult brain where they are crucial for its function [11].
GSK-3 is unique among other kinases because it is constitutively active in quiescence cells under resting conditions [12, 13]. The extracellular signals such as growth factors, neurotransmitters and hormones initiate signaling pathways, which cause the reduction of GSK-3 enzymatic activity by dynamic serine phosphorylation of GSK-3. This inhibitory regulation is achieved by a rapid and reversible N-terminal phosphorylation of Ser21 for GSK-3α and Ser9 for GSK-3β, which creates a pseudosubstrate that binds to the GSK-3 catalytic domain and prevents access of substrates to the GSK-3 active site [12, 14–17].
Phosphorylation and thus inhibition of GSK-3α/β is carried out by multiple kinases, including Akt/PKB and protein kinases A (PKA) and C (PKC) [6]. In contrast, the dephosphorylation of the N-terminal serine residue by the serine/threonine protein phosphatase 1 (PP1) and protein phosphatase 2A (PP2A) results in the activation of GSK-3 [6, 13, 15, 16].
In contrast, the positive regulation of GSK-3 is achieved by tyrosine phosphorylation: Tyr279 in GSK-3α and Tyr216 in GSK-3β. Tyrosine phosphorylation in GSK-3 occurs cotranslationally by autophosphorylation or is executed by different tyrosine kinases [18–21].
In the mouse brain, GSK-3β exists as three phosphoisotopes: double phosphorylation at Ser9 and Tyr216, single phosphorylation at Tyr216, and the nonphosphorylated isotype, the active form, i.e., phosphorylated at Tyr216 with little Ser9 phosphorylation predominating [22]. In neurons, changes in membrane electrical potential or insulin-like growth factor (IGF) treatment affect GSK-3β activity by dynamic PI3K/Akt-mediated phosphorylation and PP2A/PP2B-mediated dephosphorylation of Ser9 [23], while phospho-Tyr216 level remains unchanged [22].
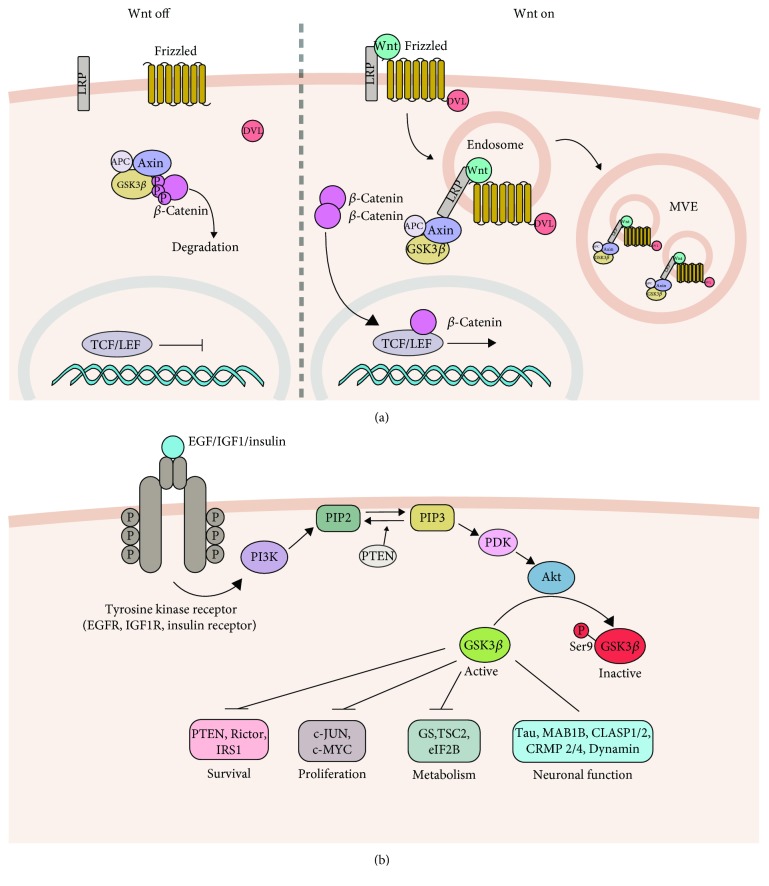
Two independently regulated pools of GSK-3 exist in the cell: the Wnt signaling pathway (Figure 1(a)) and the PI3K/Akt signaling pathway (Figure 1(b)). In the Wnt signaling pathway, in the absence of extracellular Wnt ligands or the presence of Wnt negative modulators such as extracellular protein Dickkopf-1 (DKK1), the transcriptional coactivator β-catenin is phosphorylated by GSK-3 in a complex composed of the tumor suppressor adenomatous polyposis coli (APC) and the scaffolding protein Axin. Subsequently, phosphorylated β-catenin is targeted for proteasome-dependent degradation. In the presence of extracellularly secreted Wnt proteins, Frizzled receptor and the low-density lipoprotein-related protein 5 and 6 (LRP5/6) receptors are activated [24]. This event leads to the recruitment of Dishevelled mammalian homolog Dvl1, resulting in the destabilization of the Axin-APC-GSK-3β protein complex and its sequestration into multivesicular bodies (MVB) [25]. GSK-3 inactivation allows for β-catenin stabilization and facilitates gene expression by the TCF/LEF transcription factors.
Figure 1.
Molecular mechanisms of GSK-3β regulation. (a) The Wnt canonical pathway. In the absence of Wnt, β-catenin is degraded within a destruction complex composed of Axin, APC, and GSK-3β proteins. Following Wnt binding to Frizzled and LRP5/6 receptors, Dvl is recruited resulting in the sequestration of the destruction complex within the MVB. This allows β-catenin to accumulate, translocate to the nucleus, and subsequently induce gene expression via the TCF/LEF transcription factors. (b) The PI3K/Akt pathway. The activation of PI3K following the stimulation of Tyrosine Kinase Receptor leads to the production of PIP3. Akt kinase is recruited and is activated upon phosphorylation at Thr308 and Ser473 by PDK1 and mTORC2, respectively. The signal is terminated following PIP3 dephosphorylation by PTEN phosphatase. Akt kinase phosphorylates and inhibits GSK-3β activity by a reversible phosphorylation at Ser9. An incomplete list of the GSK-3β substrates and cellular processes that it regulates is shown.
In the phosphoinositide 3-kinase (PI3K)/Akt pathway, growth signals activate the catalytic subunit of PI3K, which phosphorylates phosphatidylinositol-4,5-bisphosphate (PIP2) to produce phosphatidylinositol-3,4,5-trisphosphate (PIP3) and activates phosphoinositide-dependent protein kinase-1 (PDK-1). PDK-1 phosphorylates and thus activates the recruited serine-threonine kinase Akt/protein kinase B. Akt/PKB phosphorylates GSK-3 to inhibit its activity [6, 12, 15].
GSK-3 controls many neuronal functions by phosphorylating protein substrates involved in the regulation of gene transcription, metabolism, apoptosis, and cytoskeletal dynamics (Figure 1(b)). To ensure the proper execution of these actions, GSK-3 activity must be accurately controlled by the interplay of phosphorylation, localization, and sequestration by GSK-3-interacting proteins [6, 26, 27].
4. GSK-3 Function in the Developing and Adult Brain
4.1. Neuronal Progenitors: Proliferation and Differentiation
Neural progenitor proliferation and differentiation are regulated by multiple extracellular signals and intracellular signaling mechanisms in which GSK-3 is implicated. Early in neural development, GSK-3 functions to regulate neural progenitor self-renewal, homeostasis, and apical-basal polarity via β-catenin, Notch, FGF, and Wnt signaling [28].
Establishing neuronal polarity is a consequence of the reorganization of cytoskeletal elements after the local activation of symmetry-breaking signals. GSK-3 is a key regulator of neuronal polarity and microtubule-cytoskeleton reorganization [29, 30]. These functions are controlled by GSK-3-mediated phosphorylation of microtubule-associated proteins (MAPs), such as collapsin response mediator protein-2 (CRMP-2) [31], adenomatous polyposis coli (APC) [32], Tau [33], microtubule-associated protein 1B (MAP1B) [34], Doublecortin (DCX) [35], end-binding 1 (EB1) [36], and cytoplasmic linker-associated proteins (CLASPs) [37], and subsequent regulation of cytoskeletal dynamics. For example, APC and CLASPs promote microtubule stability and, upon phosphorylation by GSK-3, they dissociate from and destabilize microtubules [37, 38]. Therefore, polarized deposition of polarity proteins underlies asymmetric cell division which is necessary for the neurogenic division of neural progenitors. Indeed, polarized apical deposition of polarity proteins, including APC, EB1, and cadherin, is disrupted in GSK-3α/β-deleted developing cortex [28].
4.2. Neuronal Migration
Following differentiation of progenitors into neurons, GSK-3 signaling is crucial to neuronal migration. For example, removal of GSK-3α and GSK-3β in cortical excitatory neurons leads to the failure of radial migration in the cortex [39]. GSK-3 regulates neuronal migration by phosphorylating key microtubule regulatory proteins such as APC and other microtubule-associated proteins to rearrange the intracellular cytoskeleton. As mentioned before, APC is a microtubule-associated protein and is important for microtubule-based cytoskeleton dynamics [40]. When GSK-3 is inactive, APC stabilizes microtubules at the leading edge of migrating neurons [38]. When GSK-3 becomes active, it binds to and phosphorylates APC causing its dissociation from microtubules [41].
Other studies have implicated other GSK-3 interacting proteins, including β-catenin and DISC1, in neuronal migration [42–46]. DISC1/GSK-3 interaction may be particularly important for determining the transition of neural progenitor self-renewal to neuronal migration because GSK-3 binds to DISC1 during the embryonic stage (E14) when neural progenitor proliferates but dissociates from DISC1 during later embryonic stages (E18) when neuronal migration takes place [46].
4.3. Neuronal Morphology and Synaptic Development
Several lines of evidence implicate GSK-3 in the regulation of different aspects of neuronal morphogenesis, including axon growth, dendritic branching, and synaptic development. Pharmacological inhibition of GSK-3 decreases the rate of axon elongation, increases the size of growth cones [47], and disturbs polarity, leading to the formation of multiple axon-like processes in hippocampal neurons [48, 49]. Likewise, genetic elevation of GSK-3β activity causes shrinkage of dendrites, whereas GSK-3β inhibition enhances dendritic growth in vivo [50]. Another study showed that neurons with deleted GSK-3 exhibit markedly abnormally oriented basal dendrites [39].
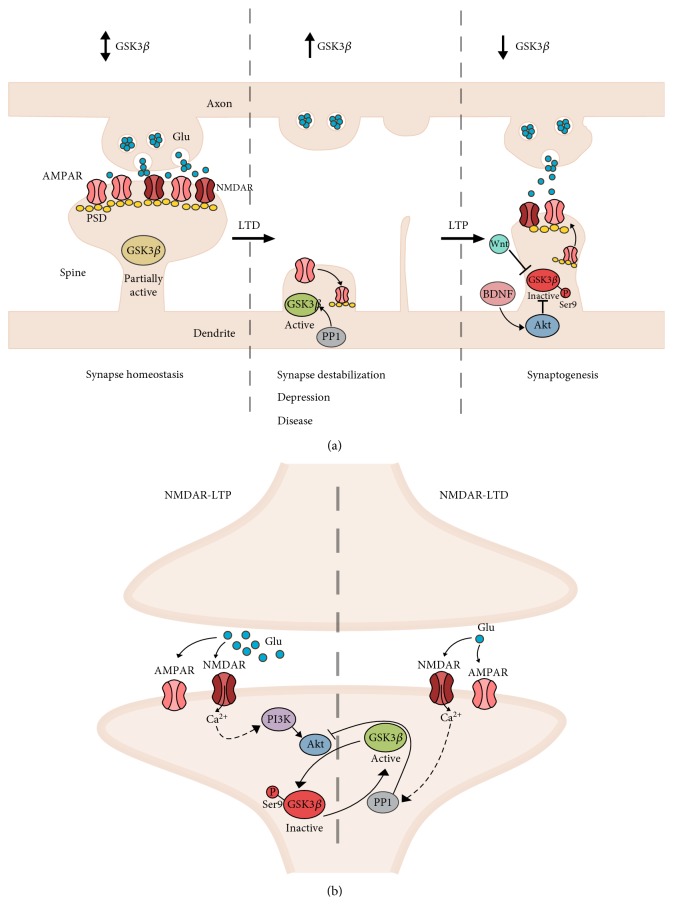
GSK-3 also contributes to the regulation of synapse morphology and formation in mature, postmitotic neurons (Figure 2(a)). Deletion of the GSK-3β gene in the cortex and hippocampus causes a reduction of spine density, loss of persistent spines, and reduced stabilization of new spines, accompanied by a decrease of AMPAR-dependent miniature excitatory postsynaptic currents [51]. Accordingly, overexpression of GSK-3β alters dendritic branching and reduces the number of the functional synapses of dentate gyrus granule neurons [52]. A recent study showed that GSK-3β is involved in the maturation of dendritic spines, because genetically elevating GSK-3β activity increases the number of thin spines, whereas removal of the GSK-3β gene increases the number of stubby spines in the dentate gyrus neurons [53]. Likewise, pharmacological inhibition of GSK-3β decreases the number of mature spines favouring an accumulation of immature types [54].
Figure 2.
GSK-3β at glutamatergic synapse. (a) Role of GSK-3β in the structural plasticity of glutamatergic synapse. (Left) Under normal conditions, synapse function is maintained by homeostatic mechanisms that depend on the cycling of glutamate receptors within the synapse. Transient changes in GSK-3β activity will support molecular mechanisms required for these processes. (Middle) Synaptic destabilization following LTD or chronic stress decreases synaptic density and causes synapse atrophy. High GSK-3β activity is required for pre- and postsynaptic molecular mechanisms to support the occurrence of LTD. Increased GSK-3β activity has been reported in different neurological and neuropsychiatric disorders. (Right) Following LTP stimuli, GSK-3β is inhibited to enable synaptic growth. LTP stimuli also increase BDNF and Wnt proteins which act to inhibit GSK-3β during LTP. (b) GSK-3β determines the direction of NMDA receptor-mediated plasticity. (Right) During LTD, activation of PP1 causes dephosphorylation and thus activation of GSK-3β by the Ser9 mechanism. Simultaneously, active PP1 inhibits Akt preventing Ser9 phosphorylation of GSK-3β. During LTP, the activation of NMDA receptors stimulates the PI3K-Akt pathway, which phosphorylates and inhibits GSK-3β activity to prevent the induction of LTD.
4.4. Neurotransmission
GSK-3α and β are present within the synapse because they were detected in the synaptosomal fraction which consists of pre- and postsynaptic termini [55]. More specifically, an electron microscopic study showed GSK-3β labelling of postsynaptic densities in a subset of dendritic spines [56].
GSK-3 plays an important role in synaptic plasticity at GABAergic as well as at glutamatergic synapses. At GABAergic synapses, active GSK-3β decreases inhibitory synaptic strength [50] by phosphorylating the scaffolding protein gephyrin [57].
At glutamatergic synapses, GSK-3β regulates the interaction between two major forms of synaptic plasticity: NMDA-dependent LTP and LTD (Figure 2(b)). During LTP, the activation of NMDA receptors causes the inhibition (by Ser9 phosphorylation) of GSK-3β activity via the PI3K/Akt pathway, whereas the action of PP1 in LTD causes an increase of GSK-3β activity [58]. Thus, GSK-3β is crucial for the initiation of NMDA-induced LTD in hippocampal neurons.
Molecular mechanisms requiring the modulation of GSK-3 Ser21/9 phosphorylation, during experimental LTP or LTD, are crucial for learning and memory [55, 58, 59]. The phosphorylation of GSK-3β at Ser9 increases following the training of mice in hippocampus-dependent cognitive tasks, i.e., inhibitory avoidance and novel object recognition task [59]. Furthermore, LTP is impaired, whereas LTD is facilitated, in two different transgenic mice overexpressing active GSK-3β [55, 59]. These LTP deficits can be reversed by treatment with lithium, a GSK-3 inhibitor [55]. Accordingly, removal of GSK-3β in dentate gyrus excitatory neurons inhibits hippocampal synaptic transmission and reduces levels of NMDAR and AMPAR receptors, postsynaptic PSD93 and drebrin, and presynaptic synaptophysin proteins causing impairments in spatial and fear memories [60].
Furthermore, GSK-3 contributes to NMDA and AMPA receptor trafficking and function in cortical neurons [61, 62]. GSK-3 causes internalization of NMDARs and forms a complex between AMPARs, thereby affecting the expression of LTD. AMPA receptor mobilization is important for LTD to occur. A critical step in this process is the destabilization of PSD-95 by GSK-3β [63].
In addition to postsynaptic actions, GSK-3 also participates in presynaptic functions in developing and mature synapses [64]. For example, high GSK-3 activity reduces glutamate release from the presynapse causing impairments in LTP [55, 65]. Additionally, retrieval of synaptic vesicles at the presynapse by endocytosis requires the regulation of dynamin 1 by GSK-3 [66]. Moreover, GSK-3β negatively regulates synaptic vesicle fusion events via interfering with Ca(2+)-dependent SNARE complex formation which is required for efficient neurotransmitter release [67]. These observations show that GSK-3 is crucial for synapse assembly and function, although the GSK-3 synaptic phosphoproteome has not been described yet. Overall, GSK-3 regulates neuronal excitation/inhibition balance. Dysregulated excitatory/inhibitory control has been reported in different neuropsychiatric disorders.
5. Implications of GSK-3 Dysregulation
5.1. GSK-3 Knockout and Transgenic Mouse Models
The dysfunction of GSK-3 signaling pathways is associated with the pathogenesis of different neurological and neuropsychiatric disorders. Several mouse models lacking or overexpressing GSK-3α or β have been generated that mimic pathological conditions observed in different neuropsychiatric and neurological disorders. These mice recapitulate pathological conditions with aberrant GSK-3 activity and thereby point at GSK-3 as a critical regulator of different physiological neurological processes.
Total removal of GSK-3β is lethal in late embryonic development due to liver apoptosis or heart defects [9, 10]. Removal of only one GSK-3β allele causes behavioral abnormalities, including aggressive behaviors, increased anxiety, and memory deficits, in GSK-3β heterozygous (+/-) mice [68, 69].
In contrast, homozygous mice lacking GSK-3α are viable but male mice are infertile [70]. They show minor abnormalities in brain anatomy, such as an altered neuronal architecture of the hippocampus [70] or a lower number of Purkinje cells in the cerebellum [71]. These two mouse strains show minor neurobehavioral abnormalities such as reduced exploratory activity, increased anxiety, and decreased social motivation and associative memory [70, 71].
Postnatal neuronal specific GSK-3β knockout mice (GSK-3βn-/-) together with GSK-3α mice (GSK-3αn-/-) were developed based on the Cre/loxP system to omit the developmental problems of GSK-3β deficiency [72]. Neurological examination showed that GSK-3βn-/- mice have reduced dentate gyrus volume [73] and decreased stability of dendritic spines [53], while GSK-3αn-/- mice have a reduced-size CA1 pyramidal blade and pre- and postsynaptic deficits [70], suggesting distinct synaptic functions of GSK-3 isozymes in the adult brain.
Transgenic mice that overexpress human GSK-3β employ the S9A mutant form of the kinase to prevent its inhibitory phosphorylation [74]. The thy1 gene promoter employed drives the expression of GSK-3β(S9A) postnatally in neurons. This transgenic mouse displays a twofold higher GSK-3β level and activity relative to wild-type mice. Consequently, increased tau phosphorylation is evident, but only in older GSK-3β(S9A) mice. These mice have decreased brain weight and volume counterbalanced by a higher cortical neuronal density and decreased size of their cell bodies and of their somatodendritic compartments [75]. The decreased brain size was further confirmed in a recent study showing a decreased dentate gyrus volume in GSK-3β(S9A) mice [73]. Biochemical analysis showed increased brain-derived neurotrophic factor (BDNF) and Akt1 levels in the hippocampus and decreased levels of PPP2R3A (PP2A regulatory subunit) and GSK-3α in the striatum in GSK-3β(S9A) mice [76]. Furthermore, overexpression of GSK-3β was shown to result in the differential expression of a large number of proteins, including the downregulation of MAP2 [77]. Despite increased tau phosphorylation and decreased hippocampus volume, GSK-3β(S9A) mice display normal memory in the Morris water maze test [74]. However, follow-up studies demonstrated impairments in hippocampal-dependent, species-typical behavioral tasks [73] and passive inhibitory avoidance [59]. Furthermore, GSK-3β(S9A) mice show hyperactivity and lower immobility time in the forced swim test (FST) which recapitulate symptoms of schizophrenia or the manic phase of bipolar disorder [76].
These mouse studies show that while GSK-3β is important during development, in the adult brain both GSK-3 isozymes have important nonredundant functions in the regulation of learning, memory, and behavior, which may result from similar but not the same spectrum of protein substrates in neurons [78].
Altogether, a delicate balance of GSK-3β activity is important for the regulation of different aspects of neuronal plasticity at the developmental stage as well as in adulthood. Not surprisingly, the dysregulation of GSK-3β activity may have deleterious consequences leading to brain disorders.
5.2. Alzheimer's Disease
Alzheimer's disease (AD) is characterized by a progressive loss of episodic memory and by cognitive and behavioral impairments and ultimately death. Synaptic dysfunction and hence memory impairments come early in the disease process. Histopathological hallmarks at postmortem analysis are extracellular senile plaques made up of amyloid-β (Aβ) protein and intracellular neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau protein. Since its initial discovery as a tau protein kinase [79], GSK-3β is considered to be essential to AD pathogenesis [80]. It plays a fundamental role in pathological events such as Tau phosphorylation, Aβ formation, neurotoxicity, neuritic dystrophy, impaired cognition, neuronal survival, and neurodegeneration [74, 81–84]. Increased levels of GSK-3 have been reported in brains from AD patients compared to age-matched control samples [85]. Furthermore, a spatial and temporal pattern of increased active GSK-3β expression correlates with the progression of neurofibrillary tangles (NFT) composed of hyperphosphorylated forms of Tau, Aβ formation, inflammatory markers, and neurodegeneration [86]. Accordingly, increased GSK-3β activity has been used to replicate neuronal dysfunctions in mouse models of AD [74, 84]. GSK-3β has been shown to be the major tau kinase in vivo [74], and it phosphorylates at least 36 residues in tau protein [87]. Furthermore, comparative phenotypic analysis of two bigenic mouse lines APP.V717I-tau.P301L and GSK-3β.S9A-tau.P301L reveals that amyloid or GSK-3β leads to a similar tau phosphorylation pattern and NFT accumulation [84]. Additionally, Aβ has been shown to activate GSK-3β signaling in vitro [88]. Altogether, GSK-3β is the mediator of amyloid action on tau phosphorylation and neurodegeneration in AD.
It should be noted that changes in the GSK-3β kinase activity, besides being involved in the regulation Aβ or tau phosphorylation, will negatively affect synaptic plasticity essential for learning and memory [55, 58, 59, 65]. For example, overexpression of GSK-3β in transgenic mice impairs memory [89, 90]. Pharmacologically balancing normal levels of GSK-3β activity recues memory deficits [90, 91]. This GSK-3β-induced cognitive impairment is mediated by tau protein because the genetic deletion of tau as well as GSK-3β inhibition blocks Aβ-induced impairments of LTP [92]. Furthermore, the genetic deletion of tau in GSK-3β-overexpressing mice ameliorates memory deficits [93].
5.3. Parkinson's Disease
Parkinson's disease (PD), the second most common neurodegenerative disease, is a chronic movement disorder resulting from the progressive loss of dopaminergic neurons in the substantia nigra pars compacta, leading to pathological and clinical abnormalities, including bradykinesia (slowness and minimal movement), rigidity, resting tremor, and postural instability. Additional symptoms include cognitive decline, depression, anxiety, and sleep disturbances resulting from neurodegeneration in the cortex and brainstem [94, 95]. The loss of dopaminergic neurons and thus decreased dopamine levels in the striatum is accompanied by an intracellular buildup of alfa-synuclein inclusions called Lewy bodies (LB) and hyperphosphorylated tau [96].
Evidence for GSK-3β involvement in PD comes from genetic studies in which single-nucleotide polymorphisms (SNPs) in the GSK-3β gene (rs334558 and rs6438552) are associated with PD [97]. The T allele (rs6438552) alters the GSK-3β splicing pattern resulting in the augmentation of GSK-3β activity [97]. Other studies in different populations have also linked SNPs in the GSK-3β gene to PD [98–100].
Accordingly, an increased GSK-3β expression has been reported in postmortem PD brains [101]. Furthermore, GSK-3β colocalizes with α-Synuclein in the Lewy bodies (LBs) [101]. In vitro GSK-3β phosphorylates α-Synuclein at Ser129 facilitating its toxic misfolding, aggregation, and accumulation leading to the degeneration of dopaminergic neurons [102]. Furthermore, GSK-3β contributes to Tau pathology associated with PD [102], corroborating the genetic data [100, 103]. Specifically, in a cell model of PD alpha-synuclein, pSer396/404-Tau and pGSK-3β coimmunoprecipitate following MPP(+) treatment [104]. Moreover, GSK-3β inhibitors prevent MPP(+)-induced death, increased α-synuclein accumulation, and pTau formation [104]. Studies from animal models demonstrated that in mice expressing a constitutively active, human GSK-3β(S9A) mutated form, levels of p-α-synuclein-S129 and pTau (S396/404) rise in TH+ dopaminergic neurons along with animal aging [102]. In α-synuclein A53T mutant mice, elevated levels of α-synuclein together with increased levels of pTau (pSer202, 396/404) and the active form of pGSK-3β (pTyr216) were detected in the striatum by western blot analysis; all of these components were also found to aggregate together, as confirmed by immunohistochemical stainings [105].
In line with these results, GSK-3β inhibitors were considered to counteract the degeneration of dopaminergic neurons. Accordingly, chronic treatment with lithium prevented the degeneration of dopaminergic neurons in the mouse model of PD [106]. Likewise, more specific GSK-3β inhibitors such as indirubin-3′-oxime and AR-A014418 suppress the loss of dopaminergic neurons and restore dopamine concentration [107].
Cautiously, human study demonstrated that chronic lithium treatment itself can induce parkinsonian pathological features, including impaired motor coordination accompanied by neuronal loss in the basal ganglia [108]. Therefore, considerations such as designing specific GSK-3 inhibitors, preventing their side effects, and determining optimum levels of GSK-3β inhibition have to be taken into account in planning GSK-3-based therapeutic strategies.
5.4. Lithium: GSK-3 Inhibitor
For many years, lithium has been used as a mood stabilizer in the treatment of mental disorders, including bipolar disorder, schizophrenia, and depression. Despite that many molecular targets have been identified, lithium is best known as a GSK-3 inhibitor [109, 110]. Lithium directly inhibits GSK-3α and GSK-3β [109] both in cells [110] and in the brain in vivo [111] at an IC50 of 2 mM, which is slightly higher than the therapeutic concentration of 0.5-1.5 mM [109]. The direct mechanism by which Li+ ions inhibit GSK-3 is that they compete for the binding of magnesium, which is a cofactor of different kinases, including GSK-3 [112]. Lithium can also indirectly inhibit GSK-3 by activating the Akt kinase or by disrupting the β-arrestin complex [113–115].
A large number of studies on the effects of lithium confirmed that GSK-3 is associated with different diseases, including fragile X syndrome (FXS) and schizophrenia. Lithium or the specific pharmacological modulation of GSK-3 activity has been shown to correct behavioral deficits in mouse models of these diseases [116, 117]. This highlights GSK-3 as a valid target of lithium; however, it must be noted that lithium is a nonspecific GSK-3 inhibitor (it inhibits many other kinases) with high in vivo toxicity.
5.5. Fragile X Syndrome
Patients with FXS have intellectual disability. Fragile X syndrome (FXS) results from the lack of expression of the functional fragile X mental retardation protein (FMRP) due to the expansion of CGG triplets resulting in the overmethylation of the gene promoter. FMRP is an RNA-binding protein that controls cellular mRNA translocation.
Since mRNA translocation towards dendrites and local translation play a pivotal role in neuronal function, FXS is characterized by several behavioral and brain structural abnormalities. Mice lacking the FMRP expression (FMRP KO mice), which model FXS, display similar characteristics as patients with FXS. FMRP KO mice exhibit impaired structural synaptic plasticity characterized by an increased dendritic spine length and number, accompanied by a reduced maturation of spines, as compared to control mice [118–120]. Indeed, other reports showed that FMRP plays a role in the normal maturation of synaptic connections [118, 121]. In addition, FX mice display distinct functional synaptic alternations such as enhanced metabotropic glutamate receptor- (mGluR-) dependent long-term depression (LTD) in the hippocampal CA1 neurons. Interestingly, further research showed aberrant mGluR signaling to GSK-3 in FX mice, and lithium treatment normalized increased mGluR-dependent LTD at CA1 synapses in these mice [122].
GSK-3 inhibition following the administration of lithium or more specific inhibitors in these mice led to corrections of multiple functional and structural FX-related phenotypes, such as normalization of hyperactive locomotor and social behaviors and improvement of passive avoidance learning as well as normalization of dendritic spine length and density and synaptic transmission [116, 123].
5.6. Schizophrenia
Schizophrenia is a widespread mental disorder, characterized by progressive functional decline and lifelong disability. Common symptoms are typically categorized into positive (hallucinations and delusions), negative (disruption of normal emotions and behavior), and cognitive (disruption of executive performance and memory). People with schizophrenia often have additional mental health problems such as anxiety or depression. Schizophrenia is thought to be caused by a combination of environmental and genetic factors.
Genetic studies have supported the association between AKT1 genetic variants and schizophrenia [124, 125], suggesting that impaired AKT/GSK-3 signaling contributes to the pathogenesis of schizophrenia [125, 126]. AKT1 protein level is significantly reduced in the hippocampus and frontal cortex in postmortem brain samples. Consequently, the activity of the major AKT1 target—GSK-3—is altered in patients with schizophrenia [125]. Additionally, GSK-3β promoter polymorphism rs3755557 that results in a higher promoter activity [127] is associated with schizophrenia in the Chinese population [128].
A recent study showed that increased GSK-3β activity early in development predisposes to altered synaptic plasticity, dendritic spine loss, and cognitive disability in a rat neurodevelopmental model of schizophrenia [54]. Accordingly, chronic treatment with antipsychotics such as clozapine, risperidone, or haloperidol increases the inhibitory phosphorylation of GSK-3β in the rat prefrontal cortex and striatum [129, 130].
Dysregulated dopamine neurotransmission is thought to underlie schizophrenia pathophysiology as dopamine D2 receptor antagonists are antipsychotic drugs. Akt/GSK-3 signaling is important for dopamine D2 receptor function, because mice lacking GSK-3β have an impaired function of the striatal D2 receptor [131]. Molecularly, the D2 receptor stimulates the formation of a signaling complex made up of β-arrestin-2, Akt, and PP2A—the latter inactivates Akt by the dephosphorylation of its regulatory Thr308 residue [132]. Accordingly, the regulation of Akt by dopamine is impaired in mice devoid of β-arrestin-2 [132]. Akt inhibition is known to activate GSK-3, suggesting that GSK-3 signaling is involved in the regulation of dopamine-dependent locomotor behavior. Likewise, pharmacological or genetic abolishing of GSK-3 activity decreases dopamine-dependent locomotor behavior [133].
5.7. Major Depressive Disorder
Major depressive disorder (MDD) is the most frequent psychiatric disorder with a prevalence of 17% in the general population, although gender disproportion exists [134]. MDD negatively affects personal life and general health. The most widely used animal model of depression is the chronic unpredictable mild stress (CUMS) model in rats. CUMS results in the augmentation of GSK-3β activity [135–137]. Accordingly, lithium and specific GSK-3β inhibitors ameliorate cognitive deficits induced by CMS [135–137].
One of the associated symptoms of MDD are disturbances in the hypothalamic–pituitary–adrenal axis (HPA axis) connected with an incorrect response of the glucocorticoid receptor to chronic stress [138]. Chronic administration of corticosterone that models depression in mice impairs synaptic plasticity and upregulates GSK-3β activity—both of which are ameliorated by the administration of an antidepressant drug [139].
The GSK-3β gene may have a role in determining regional grey matter (GM) volume differences in MDD. Analysis of single-nucleotide polymorphisms (SNPs) of GSK-3β with regional GM volume differences in patients with MDD showed the most significant association for rs6438552 [140]. In a different study, the activating allele T of the functional polymorphism rs334558 was significantly associated with remission in MDD [141].
5.8. Bipolar Disorder
Bipolar affective disorder is characterized by manic episodes that are interspersed with depression. Inadequate serotonin (5HT) neurotransmission may be a key factor driving depression. Evidence suggests that increased serotonergic activity following the administration of antidepressants inhibits GSK-3β in the brain by the Ser9 mechanism [142]. Thus, GSK-3β may not be properly inhibited in conditions of decreased 5HT levels in depression. Indeed, lower phosphorylated GSK-3β Ser9 levels were detected in platelets of patients with schizophrenia [143]. Indeed, animal studies provide further support that overactive GSK-3 contributes to depression. Transgenic mice with GSK-3β overexpression show increased locomotor activity as seen in the manic phase of bipolar disorder [76]. Furthermore, the administration of the GSK-3β peptide inhibitor, ATP competitive inhibitor, and lithium and the genetic reduction of GSK-3β in GSK-3β+/- mice produce antidepressant behavioral effects, such as decreased immobilization time in FST, which is indicative of depressive behavior [69, 144, 145].
5.9. Epilepsy
Epilepsy, which is estimated to affect over 50 million people worldwide, comprises a group of neurological diseases characterized by epileptic seizures resulting from an excessive neuronal activity [146]. In addition to seizures, epilepsy is usually associated with cognitive impairments. Epilepsy frequently accompanies various mental conditions, such as autism spectrum disorders or schizophrenia. Development of epilepsy, known as epileptogenesis, may take months or even years following brain injury, stroke, brain tumors, brain infections, or birth defects, whereas a small proportion of the cases are due to genetic mutations [147, 148]. Epileptogenesis can be reproduced in animal models using electrical or chemical kindling with pentylenetetrazole (PTZ), whereas the status epilepticus is induced by kainic acid (KA) or pilocarpine [149]. Even though extensive research shows that GSK-3 contributes to brain excitability and seizure-induced pathology, the existing data are conflicting [150–153]. For example, GSK-3β phosphorylation at Ser9 was reported to increase or decrease in brain tissue extracted from epileptic patients [154, 155]. Furthermore, kainic acid- (KA-) triggered epileptogenesis was shown to either increase or inhibit GSK-3β activity [152, 156]. Acute PTZ injection rapidly increases GSK-3β Ser9 phosphorylation and PTZ-induced kindling also gradually increases phosphorylation at Ser9 [53, 151], whereas pilocarpine-induced seizures transiently inactivate GSK-3β [150]. Pharmacological studies aimed at elucidating the role of GSK-3β inhibition in epilepsy showed a neuroprotective effect of GSK-3β inhibition against glutamate-induced toxicity in vitro and in vivo [157]. Accordingly, the GSK-3β inhibitor TDZD-8 protects against seizure-induced damage [152]. Consistently, a recent study reported the anticonvulsant properties of two distinct GSK-3 inhibitors (Indirubin and BIO-acetoxime) in three different animal models of epilepsy: the PTZ-treated zebrafish, the pilocarpine rat model for limbic seizures, and the 6 Hz refractory seizure mouse model [158]. In contrast, lithium was shown to exert proconvulsive [159] or anticonvulsive effects [160].
More complexity comes from recent animal studies. It was shown that genetically increasing as well as decreasing the activity of GSK-3β exacerbated seizure-induced brain damage after KA injection into the amygdala [161]. In a different study, GSK-3β decreased the susceptibility to kainic acid-induced epileptiform discharges and the progression of kainic acid-induced epileptogenesis [162]. Similarly, the neuronal deficiency of GSK-3β exacerbated the magnitude and severity of PTZ-induced seizures in GSK-3βn-/- mice (with postnatal neuronal deficiency) [53].
Regardless of these discrepancies, GSK-3β is considered an important contributor to the development of epilepsy.
6. Conclusions
Evidence convincingly shows that GSK-3β is critically involved in various aspects of brain function starting from early brain development, to distinct aspects of its function in the adult such as proper synaptic development and neurotransmission. GSK-3β is regulated at multiple levels and precise balance of its activity is important to execute its functions in neurons. Not surprisingly dysregulation of GSK-3β activity either in the early development or in the adulthood may predispose to neuropsychiatric and neurological disorders. GSK-3β is thus a relevant target for treatment of these diseases. Few GSK-3 inhibitors are currently undergoing clinical trials for various disorders such as progressive supranuclear palsy, Alzheimer's disease or cancer [163]. Pharmacological targeting of this kinase, however, may be problematic because of its involvement in different signaling pathways as well as because of overlapping functions with GSK-3α isozyme. Therefore, generating novel inhibitors with increased specificity, designing co-treatments and preventing side effects are of importance in pharmacological targeting of GSK-3.
Acknowledgments
This study was supported by the National Science Centre grant 2015/17/B/NZ3/03734.
Conflicts of Interest
All authors declare that they have no conflict of interest.
References
- 1.Malinow R., Malenka R. C. AMPA receptor trafficking and synaptic plasticity. Annual Review of Neuroscience. 2002;25(1):103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 2.Lisman J. Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1715, article 20160260) doi: 10.1098/rstb.2016.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao R., Penzes P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Current Molecular Medicine. 2015;15(2):146–167. doi: 10.2174/1566524015666150303003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penzes P., Cahill M. E., Jones K. A., VanLeeuwen J. E., Woolfrey K. M. Dendritic spine pathology in neuropsychiatric disorders. Nature Neuroscience. 2011;14(3):285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. European Journal of Biochemistry. 1980;107(2):519–527. [PubMed] [Google Scholar]
- 6.Jope R. S., Johnson G. V. W. The glamour and gloom of glycogen synthase kinase-3. Trends in Biochemical Sciences. 2004;29(2):95–102. doi: 10.1016/j.tibs.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO Journal. 1990;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alon L. T., Pietrokovski S., Barkan S., et al. Selective loss of glycogen synthase kinase-3α in birds reveals distinct roles for GSK-3 isozymes in tau phosphorylation. FEBS Letters. 2011;585(8):1158–1162. doi: 10.1016/j.febslet.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Hoeflich K. P., Luo J., Rubie E. A., Tsao M. S., Jin O., Woodgett J. R. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406(6791):86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- 10.Kerkela R., Kockeritz L., MacAulay K., et al. Deletion of GSK-3β in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. The Journal of Clinical Investigation. 2008;118(11):3609–3618. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao H. B., Shaw P. C., Wong C. C., Wan D. C. C. Expression of glycogen synthase kinase-3 isoforms in mouse tissues and their transcription in the brain. Journal of Chemical Neuroanatomy. 2002;23(4):291–297. doi: 10.1016/S0891-0618(02)00014-5. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland C., Leighton I. A., Cohen P. Inactivation of glycogen synthase kinase-3β by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochemical Journal. 1993;296(1):15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodgett J. R. Regulation and functions of the glycogen synthase kinase-3 subfamily. Seminars in Cancer Biology. 1994;5(4):269–275. [PubMed] [Google Scholar]
- 14.Eldar-Finkelman H., Seger R., Vandenheede J. R., Krebs E. G. Inactivation of glycogen synthase kinase-3 by epidermal growth factor is mediated by mitogen-activated protein kinase/p90 ribosomal protein S6 kinase signaling pathway in NIH/3T3 cells. Journal of Biological Chemistry. 1995;270(3):987–990. doi: 10.1074/jbc.270.3.987. [DOI] [PubMed] [Google Scholar]
- 15.Grimes C. A., Jope R. S. The multifaceted roles of glycogen synthase kinase 3β in cellular signaling. Progress in Neurobiology. 2001;65(4):391–426. doi: 10.1016/S0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- 16.Stambolic V., Woodgett J. R. Mitogen inactivation of glycogen synthase kinase-3β in intact cells via serine 9 phosphorylation. Biochemical Journal. 1994;303(3) Part 3:701–704. doi: 10.1042/bj3030701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang F., Phiel C. J., Spece L., Gurvich N., Klein P. S. Inhibitory phosphorylation of glycogen synthase kinase-3 (GSK-3) in response to lithium. Journal of Biological Chemistry. 2003;278(35):33067–33077. doi: 10.1074/jbc.M212635200. [DOI] [PubMed] [Google Scholar]
- 18.Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO Journal. 1993;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim L., Liu J., Kimmel A. R. The novel tyrosine kinase ZAK1 activates GSK3 to direct cell fate specification. Cell. 1999;99(4):399–408. doi: 10.1016/S0092-8674(00)81526-3. [DOI] [PubMed] [Google Scholar]
- 20.Lochhead P. A., Kinstrie R., Sibbet G., Rawjee T., Morrice N., Cleghon V. A chaperone-dependent GSK3β transitional intermediate mediates activation-loop autophosphorylation. Molecular Cell. 2006;24(4):627–633. doi: 10.1016/j.molcel.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q. M., Fiol C. J., DePaoli-Roach A., Roach P. J. Glycogen synthase kinase-3 beta is a dual specificity kinase differentially regulated by tyrosine and serine/threonine phosphorylation. Journal of Biological Chemistry. 1994;269(20):14566–14574. [PubMed] [Google Scholar]
- 22.Krishnankutty A., Kimura T., Saito T., et al. In vivo regulation of glycogen synthase kinase 3β activity in neurons and brains. Scientific Reports. 2017;7(1):p. 8602. doi: 10.1038/s41598-017-09239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee Y. I., Seo M., Kim Y., et al. Membrane depolarization induces the undulating phosphorylation/dephosphorylation of glycogen synthase kinase 3β, and this dephosphorylation involves protein phosphatases 2A and 2B in SH-SY5Y human neuroblastoma cells. Journal of Biological Chemistry. 2005;280(23):22044–22052. doi: 10.1074/jbc.M413987200. [DOI] [PubMed] [Google Scholar]
- 24.Wehrli M., Dougan S. T., Caldwell K., et al. arrow encodes an LDL-receptor-related protein essential for Wingless signalling. Nature. 2000;407(6803):527–530. doi: 10.1038/35035110. [DOI] [PubMed] [Google Scholar]
- 25.Taelman V. F., Dobrowolski R., Plouhinec J. L., et al. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doble B. W., Woodgett J. R. GSK-3: tricks of the trade for a multi-tasking kinase. Journal of Cell Science. 2003;116(7):1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frame S., Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochemical Journal. 2001;359(1) Part 1:1–16. doi: 10.1042/bj3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim W. Y., Wang X., Wu Y., et al. GSK-3 is a master regulator of neural progenitor homeostasis. Nature Neuroscience. 2009;12(11):1390–1397. doi: 10.1038/nn.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H., Guo W., Liang X., Rao Y. Both the establishment and the maintenance of neuronal polarity require active mechanisms. Cell. 2005;120(1):123–135. doi: 10.1016/j.cell.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 30.Etienne-Manneville S., Hall A. Cdc42 regulates GSK-3β and adenomatous polyposis coli to control cell polarity. Nature. 2003;421(6924):753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- 31.Yoshimura T., Kawano Y., Arimura N., Kawabata S., Kikuchi A., Kaibuchi K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi: 10.1016/j.cell.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Zhou F. Q., Zhou J., Dedhar S., Wu Y. H., Snider W. D. NGF-induced axon growth is mediated by localized inactivation of GSK-3beta and functions of the microtubule plus end binding protein APC. Neuron. 2004;42(6):897–912. doi: 10.1016/j.neuron.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Stoothoff W. H., Johnson G. V. W. Tau phosphorylation: physiological and pathological consequences. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2005;1739(2–3):280–297. doi: 10.1016/j.bbadis.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi N., Marsh P., Goold R. G., Wood-Kaczmar A., Gordon-Weeks P. R. Glycogen synthase kinase-3 phosphorylation of MAP1B at Ser1260 and Thr1265 is spatially restricted to growing axons. Journal of Cell Science. 2005;118(5):993–1005. doi: 10.1242/jcs.01697. [DOI] [PubMed] [Google Scholar]
- 35.Bilimoria P. M., de la Torre-Ubieta L., Ikeuchi Y., Becker E. B., Reiner O., Bonni A. A JIP3-Regulated GSK3 /DCX Signaling Pathway Restricts Axon Branching. Journal of Neuroscience. 2010;30(50):16766–16776. doi: 10.1523/JNEUROSCI.1362-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watanabe T., Noritake J., Kakeno M., et al. Phosphorylation of CLASP2 by GSK-3 regulates its interaction with IQGAP1, EB1 and microtubules. Journal of Cell Science. 2009;122(16):2969–2979. doi: 10.1242/jcs.046649. [DOI] [PubMed] [Google Scholar]
- 37.Akhmanova A., Hoogenraad C. C., Drabek K., et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104(6):923–935. doi: 10.1016/S0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 38.Zumbrunn J., Kinoshita K., Hyman A. A., Näthke I. S. Binding of the adenomatous polyposis coli protein to microtubules increases microtubule stability and is regulated by GSK3β phosphorylation. Current Biology. 2001;11(1):44–49. doi: 10.1016/S0960-9822(01)00002-1. [DOI] [PubMed] [Google Scholar]
- 39.Morgan-Smith M., Wu Y., Zhu X., Pringle J., Snider W. D. GSK-3 signaling in developing cortical neurons is essential for radial migration and dendritic orientation. Elife. 2014;3, article e02663 doi: 10.7554/eLife.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barth A. I. M., Caro-Gonzalez H. Y., Nelson W. J. Role of adenomatous polyposis coli (APC) and microtubules in directional cell migration and neuronal polarization. Seminars in Cell & Developmental Biology. 2008;19(3):245–251. doi: 10.1016/j.semcdb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asada N., Sanada K. LKB1-Mediated Spatial Control of GSK3 and Adenomatous Polyposis Coli Contributes to Centrosomal Forward Movement and Neuronal Migration in the Developing Neocortex. Journal of Neuroscience. 2010;30(26):8852–8865. doi: 10.1523/JNEUROSCI.6140-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chenn A., Walsh C. A. Increased neuronal production, enlarged forebrains and cytoarchitectural distortions in beta-catenin overexpressing transgenic mice. Cerebral Cortex. 2003;13(6):599–606. doi: 10.1093/cercor/13.6.599. [DOI] [PubMed] [Google Scholar]
- 43.Zechner D., Fujita Y., Hülsken J., et al. β-Catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Developmental Biology. 2003;258(2):406–418. doi: 10.1016/S0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- 44.Mutch C. A., Funatsu N., Monuki E. S., Chenn A. Beta-catenin signaling levels in progenitors influence the laminar cell fates of projection neurons. Journal of Neuroscience. 2009;29(43):13710–13719. doi: 10.1523/JNEUROSCI.3022-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mao Y., Ge X., Frank C. L., et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3β/β-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizuka K., Kamiya A., Oh E. C., et al. DISC1-dependent switch from progenitor proliferation to migration in the developing cortex. Nature. 2011;473(7345):92–96. doi: 10.1038/nature09859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Owen R., Gordon-Weeks P. R. Inhibition of glycogen synthase kinase 3β in sensory neurons in culture alters filopodia dynamics and microtubule distribution in growth cones. Molecular and Cellular Neuroscience. 2003;23(4):626–637. doi: 10.1016/S1044-7431(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 48.Garrido J. J., Simón D., Varea O., Wandosell F. GSK3 alpha and GSK3 beta are necessary for axon formation. FEBS Letters. 2007;581(8):1579–1586. doi: 10.1016/j.febslet.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 49.Gartner A., Huang X., Hall A. Neuronal polarity is regulated by glycogen synthase kinase-3 (GSK-3) independently of Akt/PKB serine phosphorylation. Journal of Cell Science. 2006;119(19):3927–3934. doi: 10.1242/jcs.03159. [DOI] [PubMed] [Google Scholar]
- 50.Rui Y., Myers K. R., Yu K., et al. Activity-dependent regulation of dendritic growth and maintenance by glycogen synthase kinase 3β. Nature Communications. 2013;4(1) doi: 10.1038/ncomms3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochs S. M., Dorostkar M. M., Aramuni G., et al. Loss of neuronal GSK3β reduces dendritic spine stability and attenuates excitatory synaptic transmission via β-catenin. Molecular Psychiatry. 2015;20(4):482–489. doi: 10.1038/mp.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Llorens-Martín M., Fuster-Matanzo A., Teixeira C. M., et al. GSK-3β overexpression causes reversible alterations on postsynaptic densities and dendritic morphology of hippocampal granule neurons in vivo. Molecular Psychiatry. 2013;18(4):451–460. doi: 10.1038/mp.2013.4. [DOI] [PubMed] [Google Scholar]
- 53.Kondratiuk I., Łęski S., Urbańska M., et al. GSK-3β and MMP-9 cooperate in the control of dendritic spine morphology. Molecular Neurobiology. 2017;54(1):200–211. doi: 10.1007/s12035-015-9625-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xing B., Li Y. C., Gao W. J. GSK3β hyperactivity during an early critical period impairs prefrontal synaptic plasticity and induces lasting deficits in spine morphology and working memory. Neuropsychopharmacology. 2016;41(13):3003–3015. doi: 10.1038/npp.2016.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hooper C., Markevich V., Plattner F., et al. Glycogen synthase kinase-3 inhibition is integral to long-term potentiation. European Journal of Neuroscience. 2007;25(1):81–86. doi: 10.1111/j.1460-9568.2006.05245.x. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Costas E., Gandy J. C., Melendez-Ferro M., Roberts R. C., Bijur G. N. Light and electron microscopy study of glycogen synthase kinase-3β in the mouse brain. PLoS One. 2010;5(1):p. e8911. doi: 10.1371/journal.pone.0008911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyagarajan S. K., Ghosh H., Yevenes G. E., et al. Regulation of GABAergic synapse formation and plasticity by GSK3 -dependent phosphorylation of gephyrin. Proceedings of the National Academy of Sciences. 2011;108(1):379–384. doi: 10.1073/pnas.1011824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Peineau S., Taghibiglou C., Bradley C., et al. LTP inhibits LTD in the hippocampus via regulation of GSK3β. Neuron. 2007;53(5):703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 59.Dewachter I., Ris L., Jaworski T., et al. GSK3ß, a centre-staged kinase in neuropsychiatric disorders, modulates long term memory by inhibitory phosphorylation at Serine-9. Neurobiology of Disease. 2009;35(2):193–200. doi: 10.1016/j.nbd.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 60.Liu E., Xie A. J., Zhou Q., et al. GSK-3β deletion in dentate gyrus excitatory neuron impairs synaptic plasticity and memory. Scientific Reports. 2017;7(1):p. 5781. doi: 10.1038/s41598-017-06173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen P., Gu Z., Liu W., Yan Z. Glycogen synthase kinase 3 regulates N-methyl-D-aspartate receptor channel trafficking and function in cortical neurons. Molecular Pharmacology. 2007;72(1):40–51. doi: 10.1124/mol.107.034942. [DOI] [PubMed] [Google Scholar]
- 62.Wei J., Liu W., Yan Z. Regulation of AMPA receptor trafficking and function by glycogen synthase kinase 3. Journal of Biological Chemistry. 2010;285(34):26369–26376. doi: 10.1074/jbc.M110.121376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nelson C. D., Kim M. J., Hsin H., Chen Y., Sheng M. Phosphorylation of threonine-19 of PSD-95 by GSK-3β is required for PSD-95 mobilization and long-term depression. Journal of Neuroscience. 2013;33(29):12122–12135. doi: 10.1523/JNEUROSCI.0131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smillie K. J., Cousin M. A. The role of GSK3 in presynaptic function. International Journal of Alzheimer's Disease. 2011;2011, article 263673:1–8. doi: 10.4061/2011/263673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu L. Q., Wang S. H., Liu D., et al. Activation of glycogen synthase kinase-3 inhibits long-term potentiation with synapse-associated impairments. Journal of Neuroscience. 2007;27(45):12211–12220. doi: 10.1523/JNEUROSCI.3321-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clayton E. L., Sue N., Smillie K. J., et al. Dynamin I phosphorylation by GSK3 controls activity-dependent bulk endocytosis of synaptic vesicles. Nature Neuroscience. 2010;13(7):845–851. doi: 10.1038/nn.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu L. Q., Liu D., Hu J., et al. GSK-3 inhibits presynaptic vesicle exocytosis by phosphorylating P/Q-type calcium channel and interrupting SNARE complex formation. Journal of Neuroscience. 2010;30(10):3624–3633. doi: 10.1523/JNEUROSCI.5223-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kimura T., Yamashita S., Nakao S., et al. GSK-3β Is Required for Memory Reconsolidation in Adult Brain. PLoS One. 2008;3(10):p. e3540. doi: 10.1371/journal.pone.0003540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.O’Brien W. T., Harper A. D., Jové F., et al. Glycogen synthase kinase-3 haploinsufficiency mimics the behavioral and molecular effects of lithium. Journal of Neuroscience. 2004;24(30):6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maurin H., Lechat B., Dewachter I., et al. Neurological characterization of mice deficient in GSK3α highlight pleiotropic physiological functions in cognition and pathological activity as Tau kinase. Molecular Brain. 2013;6(1):p. 27. doi: 10.1186/1756-6606-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kaidanovich-Beilin O., Lipina T. V., Takao K., et al. Abnormalities in brain structure and behavior in GSK-3alpha mutant mice. Molecular Brain. 2009;2(1):p. 35. doi: 10.1186/1756-6606-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jaworski T., Dewachter I., Lechat B., et al. GSK-3α/β kinases and amyloid production in vivo. Nature. 2011;480(7376):E4–E5. doi: 10.1038/nature10615. [DOI] [PubMed] [Google Scholar]
- 73.Kondratiuk I., Devijver H., Lechat B., Van Leuven F., Kaczmarek L., Filipkowski R. K. Glycogen synthase kinase-3beta affects size of dentate gyrus and species-typical behavioral tasks in transgenic and knockout mice. Behavioural Brain Research. 2013;248:46–50. doi: 10.1016/j.bbr.2013.03.045. [DOI] [PubMed] [Google Scholar]
- 74.Spittaels K., Van den Haute C., Van Dorpe J., et al. Glycogen Synthase Kinase-3β Phosphorylates Protein Tau and Rescues the Axonopathy in the Central Nervous System of Human Four-repeat Tau Transgenic Mice. Journal of Biological Chemistry. 2000;275(52):41340–41349. doi: 10.1074/jbc.M006219200. [DOI] [PubMed] [Google Scholar]
- 75.Spittaels K., van den Haute C., van Dorpe J., et al. Neonatal neuronal overexpression of glycogen synthase kinase-3β reduces brain size in transgenic mice. Neuroscience. 2002;113(4):797–808. doi: 10.1016/S0306-4522(02)00236-1. [DOI] [PubMed] [Google Scholar]
- 76.Prickaerts J., Moechars D., Cryns K., et al. Transgenic mice overexpressing glycogen synthase kinase 3beta: a putative model of hyperactivity and mania. Journal of Neuroscience. 2006;26(35):9022–9029. doi: 10.1523/JNEUROSCI.5216-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tilleman K., Stevens I., Spittaels K., et al. Differential expression of brain proteins in glycogen synthase kinase-3 transgenic mice: a proteomics point of view. Proteomics. 2002;2(1):94–104. doi: 10.1002/1615-9861(200201)2:1<94::AID-PROT94>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 78.Soutar M. P. M., Kim W.-Y., Williamson R., et al. Evidence that glycogen synthase kinase-3 isoforms have distinct substrate preference in the brain. Journal of Neurochemistry. 2010;115(4):974–983. doi: 10.1111/j.1471-4159.2010.06988.x. [DOI] [PubMed] [Google Scholar]
- 79.Hanger D. P., Hughes K., Woodgett J. R., Brion J. P., Anderton B. H. Glycogen synthase kinase-3 induces Alzheimer’s disease-like phosphorylation of tau: generation of paired helical filament epitopes and neuronal localisation of the kinase. Neuroscience Letters. 1992;147(1):58–62. doi: 10.1016/0304-3940(92)90774-2. [DOI] [PubMed] [Google Scholar]
- 80.Takashima A. GSK-3 is essential in the pathogenesis of Alzheimer’s disease. Journal of Alzheimer's Disease. 2006;9(s3):309–317. doi: 10.3233/JAD-2006-9S335. [DOI] [PubMed] [Google Scholar]
- 81.Jaworski T., Dewachter I., Seymour C. M., et al. Alzheimer’s disease: old problem, new views from transgenic and viral models. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010;1802(10):808–818. doi: 10.1016/j.bbadis.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 82.Muyllaert D., Terwel D., Borghgraef P., Devijver H., Dewachter I., Van Leuven F. Transgenic mouse models for Alzheimer's disease: the role of GSK-3β in combined amyloid and tau-pathology. Revue Neurologique. 2006;162(10):903–907. doi: 10.1016/S0035-3787(06)75098-6. [DOI] [PubMed] [Google Scholar]
- 83.Muyllaert D., Kremer A., Jaworski T., et al. Glycogen synthase kinase-3β, or a link between amyloid and tau pathology? Genes, Brain and Behavior. 2008;7:57–66. doi: 10.1111/j.1601-183X.2007.00376.x. [DOI] [PubMed] [Google Scholar]
- 84.Terwel D., Muyllaert D., Dewachter I., et al. Amyloid Activates GSK-3β to Aggravate Neuronal Tauopathy in Bigenic Mice. American Journal of Pathology. 2008;172(3):786–798. doi: 10.2353/ajpath.2008.070904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pei J. J., Tanaka T., Tung Y. C., Braak E., Iqbal K., Grundke-Iqbal I. Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. Journal of Neuropathology and Experimental Neurology. 1997;56(1):70–78. doi: 10.1097/00005072-199701000-00007. [DOI] [PubMed] [Google Scholar]
- 86.Leroy K., Boutajangout A., Authelet M., Woodgett J. R., Anderton B. H., Brion J. P. he active form of glycogen synthase kinase-3? is associated with granulovacuolar degeneration in neurons in Alzheimer's disease. Acta Neuropathologica. 2002;103(2):91–99. doi: 10.1007/s004010100435. [DOI] [PubMed] [Google Scholar]
- 87.Hanger D. P., Byers H. L., Wray S., et al. Novel phosphorylation sites in tau from alzheimer brain support a role for casein kinase 1 in disease pathogenesis. Journal of Biological Chemistry. 2007;282(32):23645–23654. doi: 10.1074/jbc.M703269200. [DOI] [PubMed] [Google Scholar]
- 88.Takashima A., Noguchi K., Michel G., et al. Exposure of rat hippocampal neurons to amyloid β peptide (25–35) induces the inactivation of phosphatidyl inositol-3 kinase and the activation of tau protein kinase I/glycogen synthase kinase-3β. Neuroscience Letters. 1996;203(1):33–36. doi: 10.1016/0304-3940(95)12257-5. [DOI] [PubMed] [Google Scholar]
- 89.Lucas J. J., Hernández F., Gómez-Ramos P., Morán M. A., Hen R., Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO Journal. 2001;20(1):27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hernández F., Borrell J., Guaza C., Avila J., Lucas J. J. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3β in the brain but do not form tau filaments. J. Neurochem. 2002;83(6):1529–1533. doi: 10.1046/j.1471-4159.2002.01269.x. [DOI] [PubMed] [Google Scholar]
- 91.Engel T., Goñi-Oliver P., Lucas J. J., Avila J., Hernández F. Chronic lithium administration to FTDP-17 tau and GSK-3? overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. Journal of Neurochemistry. 2006;99(6):1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- 92.Shipton O. A., Leitz J. R., Dworzak J., et al. Tau protein is required for amyloid -induced impairment of hippocampal long-term potentiation. Journal of Neuroscience. 2011;31(5):1688–1692. doi: 10.1523/JNEUROSCI.2610-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.de Barreda E. G., Pérez M., Ramos P. G., et al. Tau-knockout mice show reduced GSK3-induced hippocampal degeneration and learning deficits. Neurobiology of Disease. 2010;37(3):622–629. doi: 10.1016/j.nbd.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 94.Selikhova M., Williams D. R., Kempster P. A., Holton J. L., Revesz T., Lees A. J. A clinico-pathological study of subtypes in Parkinson’s disease. Brain. 2009;132(11):2947–2957. doi: 10.1093/brain/awp234. [DOI] [PubMed] [Google Scholar]
- 95.Williams-Gray C. H., Evans J. R., Goris A., et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 96.Davie C. A. A review of Parkinson’s disease. British Medical Bulletin. 2008;86(1):109–127. doi: 10.1093/bmb/ldn013. [DOI] [PubMed] [Google Scholar]
- 97.Kwok J. B. J., Hallupp M., Loy C. T., et al. GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Annals of Neurology. 2005;58(6):829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- 98.Infante J., García-Gorostiaga I., Sánchez-Juan P., et al. Synergistic effect of two oxidative stress-related genes (heme oxygenase-1 and GSK3β) on the risk of Parkinson’s disease. European Journal of Neurology. 2010;17(5):760–762. doi: 10.1111/j.1468-1331.2009.02908.x. [DOI] [PubMed] [Google Scholar]
- 99.Kalinderi K., Fidani L., Katsarou Z., Clarimón J., Bostantjopoulou S., Kotsis A. GSK3β polymorphisms, MAPT H1 haplotype and Parkinson’s disease in a Greek cohort. Neurobiology of Aging. 2011;32(3):546.e1–546.e5. doi: 10.1016/j.neurobiolaging.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 100.García-Gorostiaga I., Sánchez-Juan P., Mateo I., et al. Glycogen synthase kinase-3β and tau genes interact in Parkinson’s and Alzheimer’s diseases. Annals of Neurology. 2009;65(6):759–761. doi: 10.1002/ana.21687. [DOI] [PubMed] [Google Scholar]
- 101.Nagao M., Hayashi H. Glycogen synthase kinase-3beta is associated with Parkinson’s disease. Neuroscience Letters. 2009;449(2):103–107. doi: 10.1016/j.neulet.2008.10.104. [DOI] [PubMed] [Google Scholar]
- 102.Credle J. J., George J. L., Wills J., et al. GSK-3β dysregulation contributes to Parkinson’s-like pathophysiology with associated region-specific phosphorylation and accumulation of tau and α-synuclein. Cell Death & Differentiation. 2015;22(5):838–851. doi: 10.1038/cdd.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Simón-Sánchez J., Schulte C., Bras J. M., et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nature Genetics. 2009;41(12):1308–1312. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Duka T., Duka V., Joyce J. N., Sidhu A. α-Synuclein contributes to GSK-3β-catalyzed Tau phosphorylation in Parkinson’s disease models. FASEB Journal. 2009;23(9):2820–2830. doi: 10.1096/fj.08-120410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wills J., Credle J., Haggerty T., Lee J. H., Oaks A. W., Sidhu A. Tauopathic changes in the striatum of A53T α-synuclein mutant mouse model of Parkinson’s disease. PLoS One. 2011;6(3):p. e17953. doi: 10.1371/journal.pone.0017953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Youdim M. B. H., Arraf Z. Prevention of MPTP (N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) dopaminergic neurotoxicity in mice by chronic lithium: involvements of Bcl-2 and Bax. Neuropharmacology. 2004;46(8):1130–1140. doi: 10.1016/j.neuropharm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 107.Wang W., Yang Y., Ying C., et al. Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52(8):1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 108.Lecamwasam D., Synek B., Moyles K., Ghose K. Chronic lithium neurotoxicity presenting as Parkinson’s disease. International Clinical Psychopharmacology. 1994;9(2):127–130. doi: 10.1097/00004850-199400920-00010. [DOI] [PubMed] [Google Scholar]
- 109.Klein P. S., Melton D. A. A molecular mechanism for the effect of lithium on development. Proceedings of the National Academy of Sciences. 1996;93(16):8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stambolic V., Ruel L., Woodgett J. R. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current Biology. 1996;6(12):1664–1669. doi: 10.1016/S0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- 111.Muñoz-Montaño J. R., Moreno F. J., Avila J., Diaz-Nido J. Lithium inhibits Alzheimer’s disease-like tau protein phosphorylation in neurons. FEBS Letters. 1997;411(2–3):183–188. doi: 10.1016/S0014-5793(97)00688-1. [DOI] [PubMed] [Google Scholar]
- 112.Ryves W. J., Harwood A. J. Lithium inhibits glycogen synthase kinase-3 by competition for magnesium. Biochemical and Biophysical Research Communications. 2001;280(3):720–725. doi: 10.1006/bbrc.2000.4169. [DOI] [PubMed] [Google Scholar]
- 113.Eldar-Finkelman H., Martinez A. GSK-3 inhibitors: preclinical and clinical focus on CNS. Frontiers in Molecular Neuroscience. 2011;4:p. 32. doi: 10.3389/fnmol.2011.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Freland L., Beaulieu J. M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Frontiers in Molecular Neuroscience. 2012;5:p. 14. doi: 10.3389/fnmol.2012.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jope R. S. Lithium and GSK-3: one inhibitor, two inhibitory actions, multiple outcomes. Trends in Pharmacological Sciences. 2003;24(9):441–443. doi: 10.1016/S0165-6147(03)00206-2. [DOI] [PubMed] [Google Scholar]
- 116.Franklin A. V., King M. K., Palomo V., Martinez A., McMahon L. L., Jope R. S. Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biological Psychiatry. 2014;75(3):198–206. doi: 10.1016/j.biopsych.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.King M. K., Jope R. S. Lithium treatment alleviates impaired cognition in a mouse model of fragile X syndrome. Genes, Brain and Behavior. 2013;12(7):723–731. doi: 10.1111/gbb.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Comery T. A., Harris J. B., Willems P. J., et al. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proceedings of the National Academy of Sciences. 1997;94(10):5401–5404. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Irwin S. A., Patel B., Idupulapati M., et al. Abnormal dendritic spine characteristics in the temporal and visual cortices of patients with fragile-X syndrome: a quantitative examination. Am. J. Med. Genet. 2001;98(2):161–167. doi: 10.1002/1096-8628(20010115)98:2<161::AID-AJMG1025>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 120.Irwin S. A., Idupulapati M., Gilbert M. E., et al. Dendritic spine and dendritic field characteristics of layer V pyramidal neurons in the visual cortex of fragile-X knockout mice. American Journal of Medical Genetics. 2002;111(2):140–146. doi: 10.1002/ajmg.10500. [DOI] [PubMed] [Google Scholar]
- 121.Weiler I. J., Greenough W. T. Synaptic synthesis of the Fragile X protein: possible involvement in synapse maturation and elimination. American Journal of Medical Genetics. 1999;83(4):248–252. doi: 10.1002/(SICI)1096-8628(19990402)83:4<248::AID-AJMG3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 122.Choi C. H., Schoenfeld B. P., Bell A. J., et al. Pharmacological reversal of synaptic plasticity deficits in the mouse model of Fragile X syndrome by group II mGluR antagonist or lithium treatment. Brain Research. 2011;1380:106–119. doi: 10.1016/j.brainres.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z. H., Chuang D. M., Smith C. B. Lithium ameliorates phenotypic deficits in a mouse model of fragile X syndrome. International Journal of Neuropsychopharmacology. 2011;14(05):618–630. doi: 10.1017/S1461145710000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bajestan S. N., Sabouri A. H., Nakamura M., et al. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2006;141B(4):383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- 125.Emamian E. S. AKT/GSK3 signaling pathway and schizophrenia. Frontiers in Molecular Neuroscience. 2012;5 doi: 10.3389/fnmol.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Emamian E. S., Hall D., Birnbaum M. J., Karayiorgou M., Gogos J. A. Convergent evidence for impaired AKT1-GSK3β signaling in schizophrenia. Nature Genetics. 2004;36(2):131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 127.Dobson-Stone C., Polly P., Korgaonkar M. S., et al. GSK3B and MAPT polymorphisms are associated with grey matter and intracranial volume in healthy individuals. PLoS One. 2013;8(8):p. e71750. doi: 10.1371/journal.pone.0071750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Li M., Mo Y., Luo X. J., et al. Genetic association and identification of a functional SNP at GSK3β for schizophrenia susceptibility. Schizophrenia Research. 2011;133(1–3):165–171. doi: 10.1016/j.schres.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 129.Alimohamad H., Sutton L., Mouyal J., Rajakumar N., Rushlow W. J. The effects of antipsychotics on β-catenin, glycogen synthase kinase-3 and dishevelled in the ventral midbrain of rats. Journal of Neurochemistry. 2005;95(2):513–525. doi: 10.1111/j.1471-4159.2005.03388.x. [DOI] [PubMed] [Google Scholar]
- 130.Kozlovsky N., Amar S., Belmaker R. H., Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3β protein levels in rodent frontal cortex. International Journal of Neuropsychopharmacology. 2006;9(03):337–342. doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- 131.Gomez-Sintes R., Bortolozzi A., Artigas F., Lucas J. J. Reduced striatal dopamine DA D2 receptor function in dominant-negative GSK-3 transgenic mice. European Neuropsychopharmacology. 2014;24(9):1524–1533. doi: 10.1016/j.euroneuro.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 132.Beaulieu J. M., Sotnikova T. D., Marion S., Lefkowitz R. J., Gainetdinov R. R., Caron M. G. An Akt/β-Arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 133.Beaulieu J. M., Sotnikova T. D., Yao W. D., et al. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proceedings of the National Academy of Sciences. 2004;101(14):5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Craddock N., Forty L. Genetics of affective (mood) disorders. European Journal of Human Genetics. 2006;14(6):660–668. doi: 10.1038/sj.ejhg.5201549. [DOI] [PubMed] [Google Scholar]
- 135.Silva R., Mesquita A. R., Bessa J., et al. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3β. Neuroscience. 2008;152(3):656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 136.Higuchi F., Uchida S., Yamagata H., et al. Hippocampal microRNA-124 enhances chronic stress resilience in mice. Journal of Neuroscience. 2016;36(27):7253–7267. doi: 10.1523/JNEUROSCI.0319-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Mao Q., Gong X., Zhou C., et al. Up-regulation of SIRT6 in the hippocampus induced rats with depression-like behavior via the block Akt/GSK3β signaling pathway. Behavioural Brain Research. 2017;323:38–46. doi: 10.1016/j.bbr.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 138.Moraitis A. G., Block T., Nguyen D., Belanoff J. K. The role of glucocorticoid receptors in metabolic syndrome and psychiatric illness. Journal of Steroid Biochemistry and Molecular Biology. 2017;165:114–120. doi: 10.1016/j.jsbmb.2016.03.023. [DOI] [PubMed] [Google Scholar]
- 139.Weina H., Yuhu N., Christian H., Birong L., Feiyu S., Le W. Liraglutide attenuates the depressive- and anxiety-like behaviour in the corticosterone induced depression model via improving hippocampal neural plasticity. Brain Research. 2018;1694:55–62. doi: 10.1016/j.brainres.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 140.Inkster B., Nichols T. E., Saemann P. G., et al. Association of GSK3β polymorphisms with brain structural changes in major depressive disorder. Archives of General Psychiatry. 2009;66(7):721–728. doi: 10.1001/archgenpsychiatry.2009.70. [DOI] [PubMed] [Google Scholar]
- 141.Levchenko A., Losenkov I. S., Vyalova N. M., et al. The functional variant rs334558 of GSK3B is associated with remission in patients with depressive disorders. Pharmacogenomics and Personalized Medicine. 2018;Volume 11:121–126. doi: 10.2147/PGPM.S171423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X., Zhu W., Roh M. S., Friedman A. B., Rosborough K., Jope R. S. In vivo regulation of glycogen synthase kinase-3β (GSK3β) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29(8):1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ferreira A. S., Raposo N. R. B., Sallet P. C., et al. Lower phosphorylated glycogen synthase kinase-3B levels in platelets of patients with schizophrenia: increment by olanzapine treatment. European Archives of Psychiatry and Clinical Neuroscience. 2015;265(2):167–170. doi: 10.1007/s00406-014-0505-9. [DOI] [PubMed] [Google Scholar]
- 144.Kaidanovich-Beilin O., Milman A., Weizman A., Pick C. G., Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on β-catenin in mouse hippocampus. Biological Psychiatry. 2004;55(8):781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 145.Gould T. D., Einat H., Bhat R., Manji H. K. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. International Journal of Neuropsychopharmacology. 2004;7(4):387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- 146.Fisher R. S., Cross J. H., French J. A., et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58(4):522–530. doi: 10.1111/epi.13670. [DOI] [PubMed] [Google Scholar]
- 147.Goldberg E. M., Coulter D. A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nature Reviews Neuroscience. 2013;14(5):337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Pandolfo M. Genetics of epilepsy. Seminars in Neurology. 2011;31(05):506–518. doi: 10.1055/s-0031-1299789. [DOI] [PubMed] [Google Scholar]
- 149.Becker A. J. Review: Animal models of acquired epilepsy: insights into mechanisms of human epileptogenesis. Neuropathology and Applied Neurobiology. 2018;44(1):112–129. doi: 10.1111/nan.12451. [DOI] [PubMed] [Google Scholar]
- 150.Lee C. Y., Jaw T., Tseng H. C., Chen I. C., Liou H. H. Lovastatin modulates glycogen synthase kinase-3β pathway and inhibits mossy fiber sprouting after pilocarpine-induced status epilepticus. PLoS One. 2012;7(6):p. e38789. doi: 10.1371/journal.pone.0038789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Huang W. J., Tian F. F., Chen J. M., et al. GSK-3β may be involved in hippocampal mossy fiber sprouting in the pentylenetetrazole-kindling model. Molecular Medicine Reports. 2013;8(5):1337–1342. doi: 10.3892/mmr.2013.1660. [DOI] [PubMed] [Google Scholar]
- 152.Bhowmik M., Khanam R., Saini N., Vohora D. Activation of AKT/GSK3β pathway by TDZD-8 attenuates kainic acid induced neurodegeneration but not seizures in mice. Neurotoxicology. 2015;46:44–52. doi: 10.1016/j.neuro.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 153.Gangarossa G., Sakkaki S., Lory P., Valjent E. Mouse hippocampal phosphorylation footprint induced by generalized seizures: focus on ERK, mTORC1 and Akt/GSK-3 pathways. Neuroscience. 2015;311:474–483. doi: 10.1016/j.neuroscience.2015.10.051. [DOI] [PubMed] [Google Scholar]
- 154.Talos D. M., Jacobs L. M., Gourmaud S., et al. Mechanistic target of rapamycin complex 1 and 2 in human temporal lobe epilepsy. Annals of Neurology. 2018;83(2):311–327. doi: 10.1002/ana.25149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu C., Russin J., Heck C., et al. Dysregulation of PINCH signaling in mesial temporal epilepsy. Journal of Clinical Neuroscience. 2017;36:43–52. doi: 10.1016/j.jocn.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Goodenough S., Conrad S., Skutella T., Behl C. Inactivation of glycogen synthase kinase-3β protects against kainic acid-induced neurotoxicity in vivo. Brain Research. 2004;1026(1):116–125. doi: 10.1016/j.brainres.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 157.Kelly S., Zhao H., Hua Sun G., et al. Glycogen synthase kinase 3β inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Experimental Neurology. 2004;188(2):378–386. doi: 10.1016/j.expneurol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 158.Aourz N., Serruys A.-S. K., Chabwine J. N., et al. Identification of GSK-3 as a potential therapeutic entry point for epilepsy. ACS Chemical Neuroscience. 2018;10(4):1992–2003. doi: 10.1021/acschemneuro.8b00281. [DOI] [PubMed] [Google Scholar]
- 159.Bellesi M., Passamonti L., Silvestrini M., Bartolini M., Provinciali L. Non-convulsive status epilepticus during lithium treatment at therapeutic doses. Neurological Sciences. 2006;26(6):444–446. doi: 10.1007/s10072-006-0530-1. [DOI] [PubMed] [Google Scholar]
- 160.Shukla S., Mukherjee S., Decina P. Lithium in the treatment of bipolar disorders associated with epilepsy: an open study. Journal of Clinical Psychopharmacology. 1988;8(3):201–204. doi: 10.1097/00004714-198806000-00009. [DOI] [PubMed] [Google Scholar]
- 161.Engel T., Gómez-Sintes R., Alves M., et al. Bi-directional genetic modulation of GSK-3β exacerbates hippocampal neuropathology in experimental status epilepticus. Cell Death & Disease. 2018;9(10):p. 969. doi: 10.1038/s41419-018-0963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Urbanska M., Kazmierska-Grebowska P., Kowalczyk T., et al. GSK3β activity alleviates epileptogenesis and limits GluA1 phosphorylation. EBioMedicine. 2019;39:377–387. doi: 10.1016/j.ebiom.2018.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Saraswati A. P., Ali Hussaini S. M., Krishna N. H., Babu B. N., Kamal A. Glycogen synthase kinase-3 and its inhibitors: potential target for various therapeutic conditions. European Journal of Medicinal Chemistry. 2018;144:843–858. doi: 10.1016/j.ejmech.2017.11.103. [DOI] [PubMed] [Google Scholar]