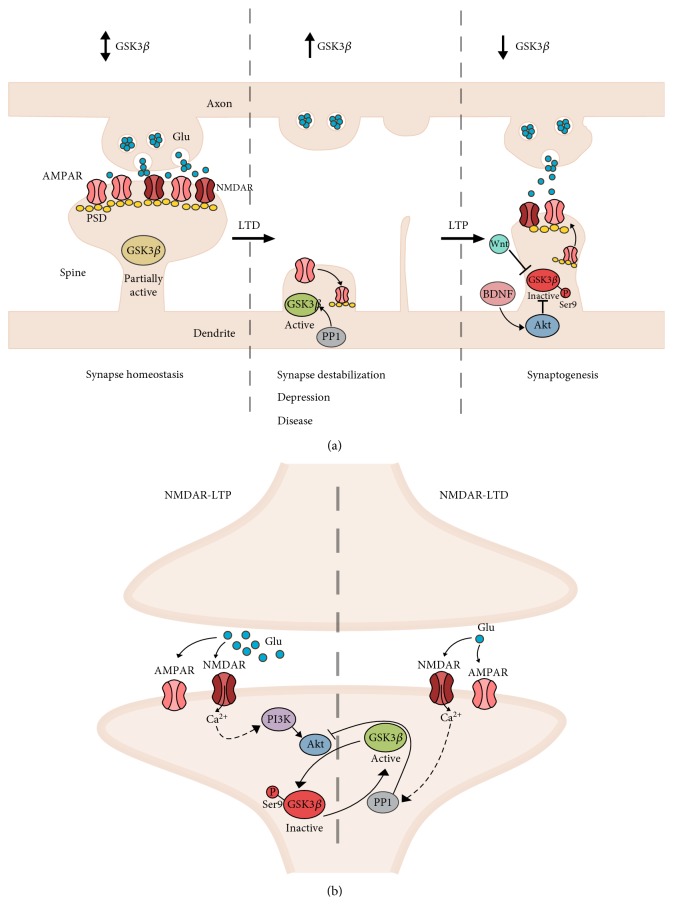
Figure 2.
GSK-3β at glutamatergic synapse. (a) Role of GSK-3β in the structural plasticity of glutamatergic synapse. (Left) Under normal conditions, synapse function is maintained by homeostatic mechanisms that depend on the cycling of glutamate receptors within the synapse. Transient changes in GSK-3β activity will support molecular mechanisms required for these processes. (Middle) Synaptic destabilization following LTD or chronic stress decreases synaptic density and causes synapse atrophy. High GSK-3β activity is required for pre- and postsynaptic molecular mechanisms to support the occurrence of LTD. Increased GSK-3β activity has been reported in different neurological and neuropsychiatric disorders. (Right) Following LTP stimuli, GSK-3β is inhibited to enable synaptic growth. LTP stimuli also increase BDNF and Wnt proteins which act to inhibit GSK-3β during LTP. (b) GSK-3β determines the direction of NMDA receptor-mediated plasticity. (Right) During LTD, activation of PP1 causes dephosphorylation and thus activation of GSK-3β by the Ser9 mechanism. Simultaneously, active PP1 inhibits Akt preventing Ser9 phosphorylation of GSK-3β. During LTP, the activation of NMDA receptors stimulates the PI3K-Akt pathway, which phosphorylates and inhibits GSK-3β activity to prevent the induction of LTD.