Abstract
Circadian rhythms are generated via variations in the expression of clock genes that are organized into a complex transcriptional–translational autoregulatory network and regulate the diverse physiological and behavioral activities that are required to adapt to periodic environmental changes. Aberrant clock gene expression is associated with a heightened risk of diseases that affect all aspects of human health, including cancers. Within the past several years, a number of studies have indicated that clock genes contribute to carcinogenesis by altering the expression of clock-controlled and tumor-related genes downstream of many cellular pathways. This review comprehensively summarizes how clock genes affect the development of tumors and their prognosis. In addition, the review provides a full description of the current state of oral cancer research that aims to optimize cancer diagnosis and treatment modalities.
Keywords: circadian rhythms, clock genes, oral squamous cell carcinoma, carcinogenesis, oral cancer
Introduction
The rotation of the earth produces a day–night cycle. Organisms align their biochemical and behavioral processes to adapt to the resulting periodic environmental changes. Most activities, such as those related to sleep–wake cycles, blood pressure, body temperature, and hormone secretion, fluctuate over an approximately 24-h period. These fluctuations, known as circadian rhythms, allow diverse physiological and behavioral activities to be coordinated and organized to benefit the survival and reproduction of organisms.1,2 Another advantage in restricting the DNA synthesis stage to the night is the reduction in DNA damage caused by strong ultraviolet radiation during the day.3 This is also a beneficial adaptation for nature in terms of the course of evolution. Circadian rhythms are affected not only by environmental cues such as light and food but also by hidden factors that comprise the “circadian clock” that controls and maintains these rhythms.1,4,5 Nearly every cell, from single-celled bacteria to those that compose fungi and mammals, including humans, possesses a self-sustained circadian clock; however, the clock differs in various species, even though it is highly conserved.5,6
The circadian clock is an intrinsic time-tracking system that is composed of central and peripheral clocks.4,7,8 The central clock is the master pacemaker of circadian rhythms and is located in the hypothalamic suprachiasmatic nucleus (SCN), which contains 10,000–15,000 neurons. Light is the only factor that influences the central clock; it is perceived by the retina and then transmitted via electrical signals to SCN neurons. These signals transmit instructions to an organism and thereby affect the peripheral clock via neurotransmitters, endocrine factors, and bodily fluids.7–9 The peripheral clock can be found in peripheral tissues such as the liver, brain, lung, heart, and kidney.8,10 Unlike the SCN, peripheral tissues seem to require a physiological stimulus to maintain circadian rhythms in mammals, and the source of this stimulus may be the SCN or SCN-mediated messages.11 Evidence suggests that restricted access to food can affect peripheral rhythms without affecting the central pacemaker function of the SCN. Thus, it is likely that the peripheral clock is hierarchically coordinated by the central clock and can also generate circadian rhythms independently.5,12 In addition, the peripheral clock could be reset in response to nonlight factors such as hormones and food.6,13 At the molecular level, the mechanisms underlying circadian rhythms generated by central and peripheral clocks are similar. There are periodic oscillations in the products of a series of clock genes that are organized into a complex transcriptional–translational autoregulatory network.7,9
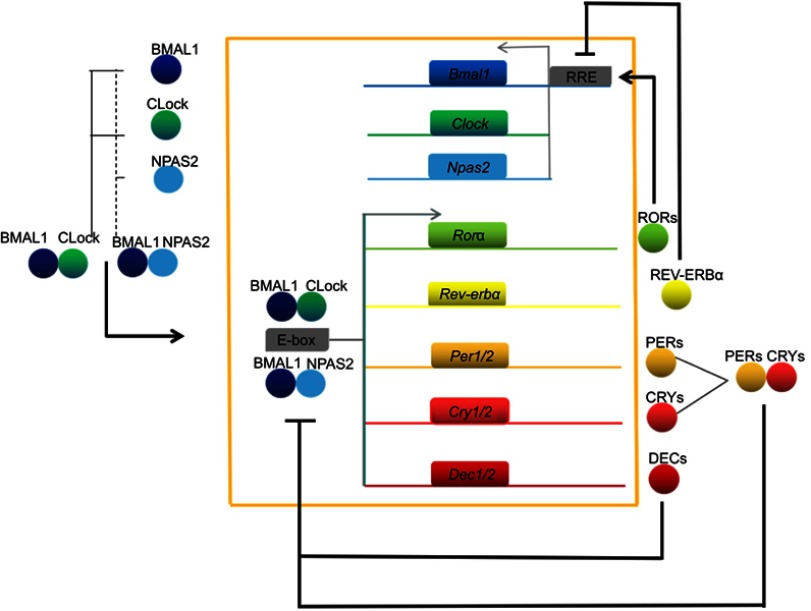
To date, the main clock genes that have been reported include Period1 (Per1), Period2 (Per2), Period3 (Per3), Cryptochrome1/2 (Cry1/2), Circadian locomotor output cycles kaput k (Clock), Brain and muscle Aarnt-like protein 1 (Bmal1; also known as ARNTL or MOP3), Casein kinase 1δ/ε (CK1δ/ε), Neuronal PAS domain protein 2 (NPAS2), nuclear receptor subfamilies (Rev-erbs, also known as NR1D), Differentiated embryo-chondrocyte expressed gene 1/2 (Dec1/2), and retinoid-related orphan receptors (Rors).5,8,14 At the molecular level, the circadian clock system is mainly composed of multiple positive and negative transcription–translation autoregulatory feedback loops that cause clock genes to oscillate for approximately 24 h and thereby produce circadian rhythms via the output system (Figure 1) (1). BMAL1 protein interacts with CLOCK or NPAS2 to produce BMAL1:CLOCK or BMAL1:NPAS2 dimers in the cytoplasm, which are then transferred to the nucleus where they recognize E-box (CACGTG) elements present in the promoters of the downstream clock genes Per, Cry, and Dec and result in transcriptional activation by binding to these sites. When PER and CRY proteins reach a certain concentration in the cytoplasm, they will be transferred to the nucleus and act on BMAL1:CLOCK or BMAL1:NPAS2 dimers, suppressing the expression of the Per and Cry genes. Meanwhile, DEC proteins can also form dimers to inhibit the transcription of the Per and Dec genes by competitively binding E-box elements via BMAL1:CLOCK or BMAL1:NPAS2, and thus achieve negative feedback regulation of circadian rhythms (2). The BMAL1:CLOCK or BMAL1:NPAS2 dimers in the cytoplasm can bind to the E-boxes in the promoters of the Rev-erbα and Rorα genes to activate transcription, and the resulting proteins can serve as transcription factors to promote and inhibit the transcription of Bmal1, respectively.8,15 Apart from the two feedback loops described briefly above, there are multiple loops that simultaneously regulate circadian rhythms.16,17 A recent study by Aryal et al18 found that PERs, CRYs, and CK1δ could be assembled into an ∼1.9-MDa repressor complex in mouse liver cell nuclei that quantitatively incorporates its CLOCK-BMAL1 transcription factor target. Indeed, many new studies have been proposed each year; however, the exact molecular mechanisms for complexing of the clock machinery remain, to a large extent, unclear.
Figure 1.
Mammalian circadian clock network. BMAL1 proteins combine with CLOCK or NPAS2 to generate BMAL1:CLOCK or BMAL1:NPAS2 heterodimers, which cause transcriptional activation of core clock genes (for example, Per, Cry, and Dec) via a combination of E-box elements; this in turn inhibits BMAL1:CLOCK/NPAS2 dimer activity. Meanwhile, the BMAL1:CLOCK/NPAS2 dimers activate the transcription of the Rev-erbα and Rorα genes, and the resulting translated proteins promote and inhibit the transcription of Bmal1, respectively.
Abbreviations: Bmal1/BMAL1, Brain and muscle Aarnt-like protein 1; Clock/CLock, Circadian locomotor output cycles kaput k; Cry/CRY, Cryptochrome; Dec/DEC, Differentiated embryo-chondrocyte expressed; Npas2/NPAS2, Neuronal PAS domain protein 2; Per/PER, Period; Rev-erb, nuclear receptor subfamily; ROR, retinoid-related orphan receptor; RRE, ROR elements.
Circadian rhythms and tumors
Studies have shown that many factors, such as drugs and radiation, can disrupt the circadian clock.6 Circadian desynchrony is correlated with a heightened risk of diseases, including obesity, depression, metabolic diseases, and cancer, that affect all aspects of human health.19–21 As the world industrializes and the pressures of living increase, the accelerated pace of the modern lifestyle causes endogenous homeostasis to be constantly disrupted by external cues in modern societies. These disruptions have been linked to a high incidence of cancers, such as lung, breast, ovarian, prostate, pancreatic, colorectal, and endometrial cancers, hepatocellular carcinoma, osteosarcoma, acute myeloid leukemia, non-Hodgkin’s lymphoma, and head and neck squamous cell carcinoma (HNSCC).5,21–23
Epidemiological investigations have also found that women workers who frequently have rotating work schedules or work at night are more prone to endometrial and breast cancers; in particular, women who work the night shift for more than 20 years may have an ~10–60% increased risk of breast cancer.24–26 This was initially discovered to be due to a decreased plasma melatonin level that resulted from light exposure at night via specialized retinal photoreceptors,27,28 since blind individuals were shown to have a lower incidence of breast cancer than the general population.29,30 However, evidence from observational studies has revealed obvious changes in circadian rhythms of cancer patients that are due not only to changes in the melatonin level but also to factors that are independent of the melatonin level.30,31 An increasing number of studies of carcinogenic risk factors have suggested that disruptions in circadian rhythms play a more central role in tumor evolution and progression than genetics.32,33 Therefore, it has been hypothesized that tumorigenesis may be related to alterations in the clock genes involved in maintaining circadian rhythms. Recent studies have revealed the phosphorylation, methylation, and the modification of histones in the promoters of clock genes in cancerous tissues that result in the deregulation of clock gene expression.14,34,35 Based on these data and the prevalence of night-shift work schedules, shift work associated with disrupted circadian rhythms was classified as a probable carcinogen by the World Health Organization in 2007.36
Disrupted circadian rhythms are not only associated with the occurrence of malignant tumors but also affect the development of cancer and the prognosis and treatment outcomes of cancer patients. In mouse models, chronic circadian misalignment has been shown to induce susceptibility to spontaneous hepatocarcinogenesis.37 Altered light–dark cycles speed the development of breast tumors and are more likely to lead to resistance during tamoxifen therapy.38 In humans, unhealthy states are usually characterized by poor circadian coordination, and the loss of circadian homeostasis could be utilized as an independent prognosis factor for survival and the therapeutic responses of patients with lung, head and neck, metastatic breast, and colorectal cancers.38–40 Therefore, the question remains: how do aberrant circadian rhythms exert an effect on the development and prognosis of tumors? This review will provide an up-to-date and comprehensive report of circadian mechanisms with a full description of the current state of oral cancer research to further optimize cancer diagnosis and treatment modalities.
Circadian clock genes and tumors
Circadian rhythms are generated via circadian variations in the expression of clock genes over an approximately 24-h period. Comparisons of transcripts in both normal and tumor cells from various organs and tissues at different time points and assayed using DNA microarray technology have revealed that almost every type of cell activity, including those involved in energy metabolism, cell division, proliferation and apoptosis, ion channels, and signal transduction, has a significant circadian rhythm.41,42 Circadian rhythms not only make complicated physiological processes synchronized and coordinated, but also allow a system to reset in response to external cues to produce improved adaptation to the environment.6,12 Notably, clock genes regulate between approximately 50% and more than 80% of the genes in the mammalian genome; these genes are known as clock-controlled genes (CCGs) and include the oncogene c-Myc and the tumor suppressor gene p53. CCGs, containing E-box regulatory elements, integrate cellular rhythmic information and encode various protein products.43,44 Circadian clock genes can participate in tumor development directly or indirectly by altering the expression of downstream CCGs involved in cell cycle regulation, DNA damage repair, cell proliferation and apoptosis, and tumor immunity.45,46
Clock genes and the cell cycle
One characteristic of malignant tumors is uncontrolled and disordered cell proliferation. This loss of control is inevitably reflected in abnormalities in cell cycle events, and the disordered nature of these cells is necessarily reflected in the disruption of their time rhythm, which exhibits a differenceof 8–12 h from normal cells.47,48 It is remarkable that circadian rhythms share some common features with the cell cycle; in addition, both are the result of the periodic and sequential activation and repression of the products of clock genes at the transcriptional, posttranscriptional, and translational levels.46,49 Alterations in circadian rhythms and the cell cycle contribute greatly to carcinogenesis.34,49
As early as the discovery of clock genes, circadian rhythms have been found in cell cycle mitosis, nucleic acid (DNA and RNA) synthesis, liver weight, glycogen content, and cell cycle protein expression in the mouse liver.50,51 As research has progressed, it has become evident that a number of cell cycle genes contain E-boxes in their promoters, and the expression of those genes, such as Cyclin A, CyclinB1, Cyclin D1, p53, and Wee1, exhibits a rhythmic pattern.52,53 The normal cell cycle is regulated by the cyclin/cyclin-dependent kinase (CDK)/cyclin-dependent kinase inhibitor (CKI) regulatory network in a precise and strict chronological order as it progresses through the G1–S–G2–M phases.54 Various cell cycle genes in mammals possess E-box regulatory elements. BMAL1:CLOCK/NPAS2 dimers recognize the E-box and produce transcriptional activation. Clock genes can influence cell cycle progression by regulating the expression of cell cycle-related genes and thus participate in the development of tumors.49
The initiation of the cell cycle is strictly controlled via an extracellular proliferation stimulus, which immediately activates the early responsive gene c-Myc that then plays an important role in the initiation of the G1 phase as well as cell growth and death.55 BMAL1:CLOCK/NPAS2 dimers can downregulate c-Myc transcription, directly or indirectly, by blocking cell cycle progression.52,55 Cell cycle checkpoints guarantee the completion of the biochemical reactions that constitute regulatory pathways.54,56 The G1 phase is the longest phase of the cell cycle, during which most biosynthesis that supports cell cycle progression occurs. Activating the G1 checkpoint causes cells to pause for a check prior to entering the S phase or exit the cell cycle to enter the G0 phase.54 The G1/S transition is negatively regulated by p21, which is one of the critical products of p53 that arrests the cell cycle; the clock genes Rev-erbα and Rorα inhibit or activate p21 expression, respectively.57 During the G2 phase, activation of the G2/M checkpoint is controlled by Wee1, which serves as an important link between the circadian clock and the cell cycle. Wee1 inhibits the activity of CDK1 to prevent inappropriate M phase entry and can be activated by BMAL1:CLOCK/NPAS2 dimers or, conversely, inactivated by PER and CRY proteins.49,51
Clock genes affect many biological pathways, including those involved in cell proliferation and apoptosis, by controlling the expression of cell cycle genes. Per1 overexpression induces c-Myc and suppresses p21 in response to ionizing radiation. Meanwhile, Per1 also inhibits the expression of Wee1, CyclinB1, CyclinD1, and CDK1, which leads to a decrease in cancer cell proliferation.58,59 Per2-deficient mice are more prone to develop cancer induced by γ irradiation and exhibit reduced p53 expression and an elevated level of c-Myc.52 Furthermore, mutations in Per1 and Per2 cause a shortening of the cell cycle and an elevation in the proliferation rate.22,52,60 It is also worth noting that the role of Per1 in some studies is in conflict with that shown in previous reports, as mentioned above, and this discrepancy may be due to differences in the methods (knockdown vs overexpression) that were used in experiments or characteristics of the cell lines that were used.61,62 Bmal1 knockout leads to cell cycle disruption and the deregulation of Wee1, CyclinB, and p21 and upregulation of CyclinE, as well as a substantial increase in the apoptotic cell population in malignant pleural mesothelioma.63 In Clock mutant mice, inhibitory cell cycle genes, including p21 and Wee1, are generally upregulated or become nonrhythmic, whereas proliferative genes, such as CDK2, CyclinD3, and CyclinE1, are downregulated.45 In Cry double-knockout mice, the expression levels of Wee1, CyclinB1, and Cdc2 are markedly deregulated, contributing to slower than normal liver regeneration.51 Despite the identification of Tim as an additional clock gene that primarily functions outside the main transcriptional–translational loops, its protein, TIM, has proven to have a checkpoint function that closely connects the circadian clock with the cell cycle.16
These data indicate that many genes crucial to the cell cycle and the phases of cell division are under the control of clock genes that are aberrantly expressed in many tumor tissues. As a consequence, personalized chronotherapy for cancer has emerged, with the aim of coordinating the time of delivery of chemotherapeutic drugs with the clock and cell cycle to optimize therapeutic efficacy while simultaneously minimizing side effects.64,65 It is well recognized that a reasonable time regimen for chemotherapeutic drugs is as important as the drug dosages that are used.66
Clock genes and DNA damage repair
In response to DNA damage, mammalian cells activate the G1/S and G2/M checkpoints of the cell cycle, during which ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) kinases, which are DNA-damage response amplification factors, phosphorylate mammalian checkpoint kinase 2 (CHK2) and CHK1, respectively, via binding to DNA-damage sites, and thus suppress CDK activity and activate CKIs to arrest the cell cycle and provide time for DNA-damage repair.67,68 Many critical players confirm circadian rhythmicity during the process of repairing damaged DNA, and P53 is the most important transcription factor among them.69 Both the ATM and CHK2 kinases phosphorylate P53, leading to P53 protein stabilization and activation. Subsequently, the activated P53 protein blocks the cell cycle progression by inducing the expression of cell cycle inhibitors such as P21, allowing the cell to enable repair or to induce apoptosis events if the DNA damage is beyond repair.69
It is noteworthy that the core clock proteins can interact with checkpoint proteins and thereby induce proliferation, apoptosis, and/or tumorigenesis. Recent studies in oral cancer cells have found that Per1 knockdown significantly increases the transcript expression levels of CyclinD1, CyclinE, CyclinB1, CDK1, and Wee1, whereas it decreases those of c-Myc, p53, and p21; PER1 protein levels modulate p53-dependent signals via the ATM and CHK2 kinases, which leads to G1/S and G2/M arrest and apoptosis.70,71 Additionally, deregulated Per2 is associated with decreased levels of WEE1, CYCLINB1, and P53, and also delays DNA damage-induced CHK2 activation, overrides DNA damage-induced apoptosis, and abolishes the gating of cell cycle entrance and G2/M arrest.72–74 When DNA replication is blocked by ultraviolet irradiation, TIM, which serves as a checkpoint protein, switches the replication checkpoint signal from ATR to Chk1, which is important for S-phase checkpoint regulation.16,75 In addition, TIM is necessary for the ATM-dependent activation of Chk2 and G2/M checkpoint arrest, and is also required for ATM activation.76 There is additional research indicating that TIM interacts with both CHK1 and CRY1 to coordinate the internal clock with the cell cycle.77 CRY1 and CRY2 have distinct responses to DNA damage in terms of protecting genomic completeness via posttranslational modification and coordinated regulation of downstream transcription.78 Additionally, the regulated degradation of DEC1 has been found to be important for G2/M checkpoint recovery via inhibition of the downregulation of p53 during the DNA-damage response.79 The Clock gene knockdown upregulates the transcript levels of p53, p21, Chk1, and Chk2 and changes the rhythmic profile of Wee1.80,81 Moreover, the Clock gene has been shown to play a direct role in the DNA-damage response as a result of its localization to the sites of DNA double-strand breaks induced by γ radiation.81 Bmal1 has been reported to exert an effect on γ-radiation-induced p53 and p53-dependent p21 activation to arrest the cell cycle upon DNA damage.82 Genetic ablations of Bmal1 and Clock lead to delayed cell cycle exit through additional rounds of cell division and high levels of cell proliferation.82 These findings suggest that many clock proteins that are closely linked to DNA damage repair during cell cycle progression are subject to the control of the circadian rhythm; however, the timing of the resulting modifications has not been defined.
Clock genes and the tumor suppressor gene p53
It is well recognized that p53 is an important anti-oncogene that safeguards genomic integrity. When DNA damage or proto-oncogenic mutation occurs, p53 receives upstream signals and triggers the stress response, which promotes the repair or apoptosis of damaged cells.69,83 The function of the anti-oncogene p53 is tightly connected with the modulation of protein stability, and the P53 protein serves as the most important transcription factor involved in diverse biological processes, including the cell cycle, apoptosis, and DNA-damage response, that are important in maintaining cell homeostasis, preventing gene mutation, and inhibiting tumor formation and development.69,84 The deletion or mutation of the 53 gene is a frequent occurrence in approximately half of all human cancers, including oral cancer.85,86
G1 phase arrest is p53 dependent and is negatively regulated by P21, which is one of the critical products of p53.87,88 PER1 can directly modulate p53-dependent signals via the ATM and CHK2 kinases to control the G1 checkpoint during responses to DNA damage. G2 phase arrest after DNA damage also requires p53 and p21, and the ATM/CHK2/p53 pathway is activated to repair the damaged DNA or initiate the apoptosis programme. The ATM/CHK2/p53 signaling pathway has been recognized as a common pathway that allows cells to cope with environmental hazards that threaten genomic stability.59
Previous research has shown that decreases in the expression of Per2 can directly inhibit the activation of the P53 protein and lead to accelerated tumor development.52 In addition, Per2 overexpression increases the mRNA and protein levels of p53, alters those of apoptosis-related genes, including c-Myc, and promotes p53-dependent G2/M arrest, all of which induce apoptotic cell death to play a role in tumor suppression.89,90 The p53 response element overlaps with the E-box and competes with BMAL1:CLOCK/NPAS2 dimers to bind to the Per2 gene promoter, and thereby indirectly participates in the regulation of the circadian clock.84,91 As we know, one p53 transcriptional targets is Murine double minute 2 (Mdm2), which encodes the E3 ubiquitin ligase MDM2 that inactivates or degrades the P53 protein. Meanwhile, MDM2 functions as an enzyme within a feedback loop that exhibits circadian variations in its expression in mouse liver.52,69 P53 and MDM2 are regulated indirectly by the Per genes via the tumor suppressor ATM at the posttranscriptional level.92 In unstressed cells, Per2 directly associates with the C-terminus region of p53 to prevent Mdm2-mediated ubiquitination of p53.91 Therefore, low levels of p53 exist at all times in the presence of Per2, and the response of p53 to genotoxic stress depends not only on its availability but also on its dissociation from Per2 in the nucleus.74 In addition, a recent study has shown that MDM2 may target PER2 for degradation, and this is important for defining the circadian period length in mammalian cells.93 The above results indicate that there is a dual-direction regulation between p53 and Per2.
It has been suggested that Bmal1 affects the capacity of p53 toward its target P21 to arrest the cell cycle upon cellular stress signals such as DNA damage, not directly modulating the activities of P21, although the exact molecular mechanism underlying its action is still unclear.58,63,82 Additionally, RORα agonists can heighten P53 protein stability and lead to increased p53 activity and subsequent apoptosis.94 Silencing the Clock gene upregulates the P53 protein level and increases apoptosis, coupled with cell cycle arrest.80 Overall, these studies support the important and multiple roles of the circadian clock in the administration of p53 pathways and have shown that the loss of p53 function in oral cancer will certainly accelerate the occurrence and development of tumors.
Clock genes and the oncogene c-Myc
The c-Myc proto-oncogene possesses E-box regulatory elements, which can be recognized by BMAL1:CLOCK/NPAS2 dimers to exert transcriptional suppression.55 c-Myc takes charge of an early responsive regulator that responds to diverse extracellular proliferation stimuli and plays an essential role in cell division, apoptosis, and metabolic pathways.55,95
When c-MYC is expressed at a high level, it tends to form a repressive complex with MIZ1 (ZBTB17) that downregulates a range of target genes.96 Ectopic c-Myc levels also allow cells with damaged DNA to progress through cell cycle checkpoints, which contributes to the genesis of many cancers in humans.55,97 Intriguingly, c-Myc can simultaneously promote the proliferation and apoptosis of tumor cells by acting via the P53-dependent proapoptotic pathway ARF/MDM2/P53 or via direct interaction with ARF.98 Although c-Myc promotes the apoptotic progression of tumor cells, it does not contradict itself as a proto-oncogene.99 Researchers speculate that c-Myc may be initially elevated and promote tumorigenesis, and then subsequently become decreased or silenced to suppress the formation of tumors, which balances cell regeneration and death during malignant cell transformation to facilitate tumor cell progression.100,101 These results also indicate the unique complexity of the function of the c-Myc gene.
MYC, as a transcription factor, can bind to almost all promoter regions within the genome and trigger widespread gene expression.102,103 Therefore, two conflicting viewpoints have been proposed to explain how MYC regulates gene expression: one posits that MYC regulates the expression of all genes,104 while the other suggests that MYC enhances the expression of specific sets of genes that, in turn, affect the expression of other genes.105 Although it is not clear which viewpoint is correct, the hypothesis that states that promoters differ in their affinity for MYC may provide an explanation regarding the differences in gene expression programmes.106
Altered expression of clock genes is likely to result in aberrant c-Myc levels, which increases the risk of cancer susceptibility. Clock gene knockout results in the downregulation of c-MYC and CYCLINB1 and the upregulation of P53, which is concomitant with the reduced proliferation and accelerated apoptosis of glioma cells.80 Loss of Cry2 facilitates the stabilization of c-MYC, and CRY2–FBXL3 cooperatively drive c-MYC ubiquitination and degradation, which limits malignant cellular transformation.107 Per1 overexpression results in an elevated level of c-MYC in colon cancer cell lines after irradiation.59 Deregulation of Per2 abolishes the oscillatory expression of c-Myc and then causes alterations in p53 function.52 BMAL1:CLOCK/NPAS2 dimers induce the transcription of the Per genes and inhibit c-Myc; therefore, PERs can indirectly suppress c-Myc transcription by regulating BMAL1:CLOCK/NPAS2 dimers.52,55 Additionally, the Per1 and c-Myc transcript levels consistently follow the same rhythmic pattern, although BMAL1:CLOCK/NPAS2 dimers regulate Per1 and c-Myc expression in an opposing fashion.108 Recent reports have highlighted the importance of the interactions between MYC and the circadian clock. MYC may repress the clock and promote proliferation via its interaction with MIZ1, thereby inducing REV-ERBα to restrain the rhythmic expression of BMAL1.95,109 In addition, the overexpression of c-MYC represses Per1 transactivation by targeting selective E-box sequences, which affects the expression of clock genes at the posttranscriptional level.110
Clock genes and tumor proliferation and apoptosis
Tumorigenesis is a very complex and diversified process. In addition to producing abnormal changes in the cell cycle, it also needs to inhibit the apoptotic signals generated via cell contact, develop new blood vessels to provide nutrition and oxygen to support tumor growth, and induce the capacity to migrate and invade.71,111–113 To date, many crucial genes involved in the cancer-related regulation of cell proliferation, apoptosis, invasion, metastasis, and angiogenesis have been documented to have periodic patterns of expression over a 24-h cycle, such as the proliferation gene Ki-67,114,115 the proto-oncogene Mdm2,52,89 the proapoptotic gene Bax, the antiapoptotic gene Bcl-2,111 the invasion and metastasis gene MMP9,116,117 and the angiogenic gene Vegf.112,114,118 These genes appear to function as CCGs that are controlled by clock genes.
The circadian clock participates in tumorigenesis by regulating downstream tumor-related genes. Per1 and Per2 have been reported to be associated with the upregulation of Ki-67, Mdm2, Bcl-2, Mmp9, and Bax and the downregulation of Bcl-2, c-Myc, and p53 in lung, mammary, pancreatic, hepatocellular, and oral carcinoma cell lines. Similar alterations related to Per2 have also been observed at the protein level. 71,89,119 The altered expression of these genes impacts tumor cell proliferation, apoptosis, migration, and invasion. At the same time, mutations in Per1 and Per2 cause a shortening of the cell cycle, an elevation of the proliferation rate in mice, attenuated proapoptotic processes, and decreased sensitivity to x-irradiation.22,52,60,120 Silencing Clock diminishes the protein levels of c-MYC and CYCLINB1 and enhances the P53 protein level, which results in increased apoptosis.80 In addition, interrogation of the mouse protein-encoding transcriptome using a DNA array assay has suggested that a number of genes that are involved in the cell cycle and cell proliferation are regulated by the Clock gene and that cell cycle inhibitory genes are generally upregulated in Clock mutant mice while pro-proliferative genes are downregulated.45 Knockdown of Dec2 has been shown to stimulate mRNA or protein expression of factors, such as c-Myc, Caspase-8, and Bax, that are correlated with apoptosis events, and overexpression of Dec2 has been shown to inhibit the proapoptotic factor BIM and reduce the amount of Caspase-8 in human breast cancer cells; however, Dec1 has been shown to exhibit opposing function.121,122 Of note, there are many signaling pathways that cooperate with clock genes to participate in tumorigenesis. For instance, BMAL1 depletion leads to cell cycle disruption, which results in a substantial increase in the apoptotic cell population and the acceleration of cell invasion by activating the PI3K/Akt/MMP2 pathway.63,123 In addition, there is a strong connection between BMAL1 regulation and the PI3K/mTOR signaling pathway, which is one of the most active pathways in human cancers.124,125 Mutations in Cry have been shown to sensitize a p53 mutant and enhance apoptosis by interfacing with the nuclear factor-κB signaling pathway.126
The occurrence and metastasis of tumors are inseparable from developmental angiogenesis. Studies suggest that the hypoxia-induced expression of Vegf plays a key role in tumor-induced revascularization, and the transcript level of Vegf was shown to exhibit circadian rhythmicity in sarcomas that were transplanted subcutaneously in mice and in cancer cells in response to hypoxia.112,114 This is because Bmal1 binds to the Vegf promoter and activates it, thereby controlling its rhythmic expression; indeed, Vegf is a direct transcriptional target of Bmal1.118,127 In contrast, Per2, Cry2, and Dec2 exert opposing angiogenic effects via the regulation of Vegf expression. The peak mRNA expression of Per2, Cry2, and Dec2 occurs at night, while that of Vegf occurs during the day.112,128 This also suggests that the selective timing of the clinical administration of antiangiogenic factors increases their efficacy.
The abnormal expression of clock genes, including Per1 and Per2, has been observed in oral cancer.86,129 As noted above, aberrant clock gene levels can alter a range of downstream CCGs and tumor-related genes, cooperating with many signaling pathways involved in cancer development.
Clock genes and tumor immunity
Studies have shown that the mammalian immune system possesses a molecular circadian clockwork that involves various populations of immune cells, such as monocytes, natural killer (NK) cells, and T and B lymphocytes, as well as the responses to signals and their defensive functions, including the levels of cytokines and other effectors, that are regulated according to a cycle of approximately 24 h.130,131 The underlying mechanism depends upon the humoral and neural regulations of the immune system by clock genes to allow immune activity to be highly ordered in space and time.131,132 When circadian homeostasis is disrupted, deregulation of the immune system alters the function of immune cells, such as NK cells, which leads to immune suppression and the accelerated development of tumors.133 In recent years, the absolute reduction of NK cells and high levels of interleukin 6 (IL-6) have been linked to faster tumor progression, higher cancer stage, and poorer prognosis for cancers in general, including HNSCC.40,134,135
BMAL1 is the central mediator of the circadian control of the immune system and promotes an anti-inflammatory state.136 Downregulation of BMAL1 has been found in hematologic malignancies such as diffuse large B-cell lymphoma, chronic lymphocytic leukemia, and acute myeloid leukemia.137 Knockout of Bmal1 affects B-cell development, which indicates a close relationship between circadian genes and immune regulation.138 In addition, the ablation or deregulation of the clock genes Per1/2, Cry1/2, Bmal1, Rev-erbα, or Clock induces an array of abnormalities in the immune system, including a reduction in proinflammatory cytokines, cytotoxic receptors, and NK and mast cell activity, as well as the inhibition of B-lymphocyte differentiation.130,132,136,139,140 Moreover, Bmal1, Clock, Rev-erbα, and Rorα have also been reported to regulate immune functions (including migration, chemotaxis, phagocytosis, and cytotoxicity) and inflammation by modulating CCGs that encode a variety of proteins, including cytokines, chemokines, and receptors.136,141
The central pacemaker controls the functioning of the immune system but is also modulated by immune factors such as proinflammatory cytokines, IL-1/6, and anti-inflammatory drugs, at the molecular, cellular, and behavioral levels, which results in subsequent alteration of the intracellular expression of Bmal1, Npas2, Cry1, and Per2.142–144
In summary, the circadian clock and the immune system exert bidirectional control.131 Disturbances in the circadian clock and the rhythmic patterns that influence neuroendocrine and immune system factors impact tumor onset and progression. Therefore, it is necessary and important to take biological time into account when using cancer chronotherapy to improve cancer therapy.
Clock genes in oral cancer
Oral cancer is a multifactorial disease; chronic tobacco use and alcohol intake are two major risk factors, while betel quid chewing, chronic inflammation, human papilloma virus infection, and genetic polymorphisms also contribute to its pathogenesis.145,146 Oral squamous cell carcinoma (OSCC) accounts for approximately 90% of malignant oral cancers and ranks as the eighth most common cancer worldwide.145,147 The majority of oral cancer patients undergoing surgery are already at an advanced stage, and despite some advances in surgical techniques and chemotherapy, the 5-year overall survival of patients is estimated to be only 50–60%.148,149 Therefore, it is indispensable to understand the basic molecular mechanisms that contribute to oral tumorigenesis to enhance preventative and therapeutic options that can be used to decrease OSCC morbidity and mortality.
As mentioned above, circadian clock genes affect tumor development and prognosis by regulating downstream CCGs that are involved in cancer-related pathways. A number of studies have reported the role of clock genes in oral carcinogenesis (Table 1). In a model of nude mice inoculated with human OSCC cells, the tumor volume and proliferative index, but not the apoptotic index, were shown to obey circadian rhythms.150 Other studies have shown that variations in the circadian patterns of the clock genes Per1 and Per2, as well as the tumor-related genes p53, c-Myc, Vegf, CyclinD1, CDK1, and CyclinB1, have been observed with the development of cancer,53,114 which indicates that carcinogenesis itself could alter circadian patterns at the cellular and molecular levels.
Table 1.
Clock genes in oral cancer
| Ref. | Cell type/origin | Methods | Results |
|---|---|---|---|
| Sato et al61 | CA9-22 cell line; human gingival cancer tissue and noncancerous tissue from 13 patients |
Knockdown and overexpression of PER1 and PER3; RT-qPCR; western blotting |
PER1 knockdown enhanced apoptosis, while PER3 knockdown inhibited apoptosis in CA9-22 cells induced by CDDP treatment; PER1 overrepresented in cancerous tissue; PER3 overrepresented in noncancerous tissue |
| Hsu et al129 | Human cancerous and noncancerous tissue from 40 HNSCC patients | RT-qPCR | PER1, PER2, PER3, CRY1, CRY2, BMAL1, and CKIε were significantly downregulated; downregulated PER3, CRY2, and BMAL1 expression was correlated with more advanced cancer stages, while downregulated PER3 correlated with larger tumor size, deeper tumor invasion, and poor survival |
| Chen et al152 | Human cancerous and healthy adjacent tissue from 41 OSCC patients | Immunocytochemistry; RT-qPCR |
Decreased expression of PER1 mRNA and protein in cancerous tissues; gradually decreased expression during cancer development |
| Zhao et al150 | 32 mice injected with human OSCC cell line BcaCD885 | Nude mouse model of OSCC |
Circadian rhythm in tumor volume and proliferative index, not in apoptotic index |
| Hsu et al151 | PB leukocytes from 94 patients with HNSCC and 56 healthy individuals | RT-qPCR; immunocytochemistry | 9 CCGs were significantly downregulated in PB leukocytes of patients with HNSCC; PER1 and CLOCK expression in PB leukocytes can be a marker for prognosis evaluation during postoperative follow-up in patients with HNSCC |
| Ye et al114 | Dimethylbenzanthracene-induced cancer, precancerous lesions, and normal tissues from 90 Syrian golden hamsters | RT-qPCR | Variations in the daily rhythm characteristics of the clock genes PER1, C-MYC, and P53 correlate with the development of cancer |
| Tan et al53 | Dimethylbenzanthracene-induced cancer, precancerous lesions, and normal tissues from 90 Syrian golden hamsters |
RT-qPCR | Variations in the daily rhythm characteristics of the clock genes PER2, P53, Cyclin D1, CDK1, and Cyclin B1 correlate with the development of cancer |
| Fu et al170 | SCC15 cell line nude mice |
PER1 knockdown; RT-qPCR; flow cytometry assay; in vivo tumorigenesis assay |
Increased expression of Cyclin D1, Cyclin E, Cyclin B1, CDK1, and WEE1; decreased expression of P53, Cyclin A2, P16, P21, and CDC25; fewer cells in S phase and more cells in G2/M phase; enhanced proliferation and reduced apoptosis; enhanced tumorigenicity in vivo |
| Li et al71 | SCC15 cell line nude mice |
PER1 knockdown; RT-qPCR; western blotting; colony formation; flow cytometry assay; cell migration/ invasion assay; in vivo tumorigenesis assay |
Increased mRNA levels of Ki-67, Mdm2, Bcl-2, Mmp2, and Mmp9; decreased c-Myc, p53, Bax, and Timp-2; proliferation, migration, and invasion increased whereas apoptosis decreased; enhanced in vivo tumerogenesis |
| Wang et al80 | Tca8113 cell line | Per2 downregulation; RT-qPCR |
Increased Cyclin A2, B1, and D1, CDK4, CDK6, and E2F1; decreased p53, p16, and p21; increased profileration; decreased apoptosis |
| Su et al153 | SCC15 cell line nude mice |
PER2 knockdown; RT-qPCR; in vivo tumorigenesis assay |
Cell proliferation, migration, and invasion markedly increased, while cancer cell apoptosis and number of cells in the G1/G0 phase reduced; enhanced in vivo tumerogenesis |
| Tang et al154 | 53 cases of TSCC tissue and adjacent noncancerous tissue specimens; 50 cases of normal tongue tissue specimens; human TSCC cell lines (SCC9, SCC25, CAL27) |
Knockdown and overexpression of Bmal1; chromatin immunoprecipitation assay; xenografted tumor model; western blotting |
Bmal1 expression was downregulated and its rhythmic pattern of expression was affected in TSCC samples and cell lines; Bmal1 inhibited cell proliferation, migration, and invasion in vitro and tumor growth in vivo; Bmal1 inhibited tumorigenesis and increased paclitaxel sensitivity, and its chronotherapy efficacy was correlated with fluctuations of Bmal1 mRNA level |
| Xiong et al86 | OSCC tissues and cancer-adjacent tissues from 8 patients; paraffin-embedded tissue sections of 40 OSCC patients |
RT-qPCR; western blotting; immunohistochemistry |
PER2, PTEN, P53, P14ARF, and Caspase-8 mRNA and protein expression levels in OSCC were significantly downregulated; reduced PER2 promoted the occurrence and metastasis of OSCC, and shortened the survival time; PER2 expression was negatively correlated with PIK3CA and P53 levels, and positively correlated with PTEN, P14ARF, and Caspase-8 levels |
Abbreviations: BMAL1, Brain and muscle Aarnt-like protein 1; CCG, clock-controlled gene; CDK, cyclin-dependent kinase; CK1ε, Casein kinase 1ε; CLOCK, Circadian locomotor output cycles kaput k; CRY, Cryptochrome; HNSCC, head and neck squamous cell carcinoma; Mdm2, Murine double minute 2; OSCC, oral squamous cell carcinoma; PB, peripheral blood; PER, Period; RT-qPCR, quantitative reverse transcription polymerase chain reaction.
A retrospective study that investigated clock gene expression in cancerous and noncancerous HNSCC tissues obtained from 40 patients has shown that the transcript levels of Per, Cry, Bmal1, and CKIε were significantly downregulated in cancerous tissues. Meanwhile, downregulation of Per3, Cry2, and Bmal1 were observed to be associated with more advanced cancer stages, while the decreased expression of Per3 was associated with larger tumor size, increased tumor invasion, and poorer survival.129 In the peripheral blood of patients with HNSCC, clock gene expression is similarly downregulated; PER1 and CLOCK are believed to be potential circulating prognostic markers for HNSCC.151 Decreased levels of Per1 mRNA and protein have been observed in cancerous tissues from OSCC patients, and PER1 protein expression has been shown to gradually decrease as tumor development progresses in terms of clinical staging and lymph node metastasis, proving, once again, the important role of Per1 in carcinogenesis.152
To clarify how altered Per1 expression affects tumor development, short hairpin RNAs were used in human OSCC cells. Per1 knockdown was shown to promote cancer cell growth, proliferation, apoptosis resistance, migration, and invasion; changes in cell cycle phase distribution in vitro; and accelerated tumorigenesis in vivo. Additionally, Per1 downregulation results in significantly increased levels of Ki-67, Mdm2, Bcl-2, Mmp2/9, CyclinD1, CyclinE, CyclinB1, CDK1, and Wee1 transcripts and decreased levels of c-Myc, p53, p16, p21, Bax, Timp-2, and CyclinA2 transcripts.70,71 These results suggest that Per1 acts as an important tumor suppressor gene by regulating tumor-related genes that function downstream of the cell cycle, proliferation, and apoptosis pathways. Nevertheless, there have been results from oral cancer studies that conflict with those from previous reports, as was previously mentioned. Per1 has been proven to have antiapoptotic effects, and Per3 has been shown to have proapoptotic effects during cisplatin treatment in human gingival cancer;61 the discrepancies may be due to the characteristics of the cell lines that were used or the different procedures that were used in the experiments.
In addition to the Per1 gene, the core clock gene Per2 has also attracted much attention. In a recent study of OSCC tissues from 8 patients and paraffin-embedded tissue sections from 40 OSCC patients, the mRNA and protein expression levels of PER2, P53, PTEN, and Caspase-8 were significantly reduced, and the downregulation of PER2 accelerated the occurrence and metastasis of OSCC and shortened the survival time of patients. Additionally, PER2 expression was negatively correlated with PIK3CA and P53 levels and positively correlated with PTEN and Caspase-8 levels, revealing associations between the PER2 and P53 PI3K/AKT pathways.86 PTEN, a tumor suppressor gene, is also a key molecular controller of the PI3K signaling pathway, and the loss of PTEN results in the activation of the BMAL1 protein, which ultimately connects the PI3K signaling pathway to the regulation of circadian rhythms.86 In addition, deregulated Per2 expression in OSCC cells leads to alterations in the cell cycle and a reduction in apoptosis, which is coupled with altered levels of downstream tumor-related genes.153 Overall, the core gene Per2 plays an extensive and complex antitumor role during tumor development and prognosis.
Circadian clock genes influence not only cancer development but also sensitivity to chemotherapy drugs. In OSCC specimens and cell lines, Bmal1 expression is decreased, and its rhythmic pattern is changed. Furthermore, Bmal1 overexpression results in an increase in the apoptotic cell population after exposure of cells to paclitaxel and enhances paclitaxel sensitivity in vivo. Other research shows that paclitaxel efficacy is influenced by the Bmal1 expression level in OSCC. Consequently, Bmal1 acts as a tumor suppressor gene that elevates the sensitivity of cancer cells to paclitaxel.154
As previously stated, the circadian clock affects a variety of cellular processes, especially cell proliferation, DNA repair, and apoptosis, and it is no surprise that monitoring circadian rhythms will be beneficial during cancer treatment. In view of the differences in circadian rhythmicity between tumor and normal tissues, the delivery of drugs during phases in which the repair rate is increased in normal tissue would be expected to improve their therapeutic effects.
Acknowledgments
The author would like to thank Peng Zhao for his editing suggestions. Meanwhile, the author is grateful to Ting Tan for her companionship and support.
Disclosure
The author declares no conflict of interest in the work presented.
References
- 1.Dobson CM. Dynamics and Timekeeping in Biological Systems. Annu Rev Biochem. 2014;83(1):159–164. doi: 10.1146/annurev-biochem-013014-102724 [DOI] [PubMed] [Google Scholar]
- 2.Chaix A, Zarrinpar A, Panda S. The circadian coordination of cell biology. J Cell Biol. 2016;215(1):15–25. doi: 10.1083/jcb.201603076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosato E, Kyriacou CP. Origins of circadian rhythmicity. J Biol Rhythms. 2002;17(6):506–511. doi: 10.1177/0748730402238232 [DOI] [PubMed] [Google Scholar]
- 4.Golombek DA, Rosenstein RE. Physiology of circadian entrainment. Physiol Rev. 2010;90(3):1063–1102. doi: 10.1152/physrev.00009.2009 [DOI] [PubMed] [Google Scholar]
- 5.Greene MW. Circadian rhythms and tumor growth. Cancer Lett. 2012;318(2):115–123. doi: 10.1016/j.canlet.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 6.Rohling JH, vanderLeest HT, Michel S, Vansteensel MJ, Meijer JH. Phase resetting of the mammalian circadian clock relies on a rapid shift of a small population of pacemaker neurons. PLoS One. 2011;6(9):e25437. doi: 10.1371/journal.pone.0025437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011;74:175–230. doi: 10.1016/B978-0-12-387690-4.00006-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821 [DOI] [PubMed] [Google Scholar]
- 10.Storch KF, Lipan O, Leykin I, et al. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417(6884):78–83. doi: 10.1038/nature744 [DOI] [PubMed] [Google Scholar]
- 11.Kramer A, Yang FC, Kraves S, Weitz CJ. A screen for secreted factors of the suprachiasmatic nucleus. Methods Enzymol. 2005;393:645–663. doi: 10.1016/S0076-6879(05)93034-6 [DOI] [PubMed] [Google Scholar]
- 12.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111(7):919–922. [DOI] [PubMed] [Google Scholar]
- 13.Eckel-Mahan K, Sassone-Corsi P. Metabolism and the circadian clock converge. Physiol Rev. 2013;93(1):107–135. doi: 10.1152/physrev.00016.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reszka E, Zienolddiny S. Epigenetic Basis of Circadian Rhythm Disruption in Cancer. Methods Mol Biol. 2018;1856:173–201. [DOI] [PubMed] [Google Scholar]
- 15.Crumbley C, Wang Y, Kojetin DJ, Burris TP. Characterization of the core mammalian clock component, NPAS2, as a REV-ERBalpha/RORalpha target gene. J Biol Chem. 2010;285(46):35386–35392. doi: 10.1074/jbc.M110.129288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazzoccoli G, Laukkanen MO, Vinciguerra M, Colangelo T, Colantuoni V. A timeless link between circadian patterns and disease. Trends Mol Med. 2016;22(1):68–81. doi: 10.1016/j.molmed.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 17.Schibler U. Mammalian circadian cogwheels are parts of macromolecular machines. Mol Cell. 2017;67(5):727–729. doi: 10.1016/j.molcel.2017.08.024 [DOI] [PubMed] [Google Scholar]
- 18.Aryal RP, Kwak PB, Tamayo AG, et al. Macromolecular assemblies of the mammalian circadian clock. Mol Cell. 2017;67(5):770–782.e776. doi: 10.1016/j.molcel.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nernpermpisooth N, Qiu S, Mintz JD, et al. Obesity alters the peripheral circadian clock in the aorta and microcirculation. Microcirculation. 2015;22(4):257–266. doi: 10.1111/micc.12192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinho M, Sehmbi M, Cudney LE, et al. The association between biological rhythms, depression, and functioning in bipolar disorder: a large multi-center study. Acta Psychiatr Scand. 2016;133(2):102–108. doi: 10.1111/acps.12442 [DOI] [PubMed] [Google Scholar]
- 21.Gery S, Koeffler HP. Circadian rhythms and cancer. Cell Cycle. 2010;9(6):1097–1103. doi: 10.4161/cc.9.6.11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao Q, Gery S, Dashti A, et al. A role for the clock gene per1 in prostate cancer. Cancer Res. 2009;69(19):7619–7625. doi: 10.1158/0008-5472.CAN-08-4199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lahti TA, Partonen T, Kyyronen P, Kauppinen T, Pukkala E. Night-time work predisposes to non-Hodgkin lymphoma. Int J Cancer. 2008;123(9):2148–2151. doi: 10.1002/ijc.23566 [DOI] [PubMed] [Google Scholar]
- 24.Hansen J. Increased breast cancer risk among women who work predominantly at night. Epidemiology. 2001;12(1):74–77. [DOI] [PubMed] [Google Scholar]
- 25.Schernhammer ES, Laden F, Speizer FE, et al. Rotating night shifts and risk of breast cancer in women participating in the nurses‘ health study. J Natl Cancer Inst. 2001;93(20):1563–1568. [DOI] [PubMed] [Google Scholar]
- 26.Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. 2001;93(20):1557–1562. [DOI] [PubMed] [Google Scholar]
- 27.Stevens RG, Rea MS. Light in the built environment: potential role of circadian disruption in endocrine disruption and breast cancer. Cancer Causes Control. 2001;12(3):279–287. doi: 10.1023/A:1011237000609 [DOI] [PubMed] [Google Scholar]
- 28.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- 29.Flynn-Evans EE, Stevens RG, Tabandeh H, Schernhammer ES, Lockley SW. Total visual blindness is protective against breast cancer. Cancer Causes Control. 2009;20(9):1753–1756. doi: 10.1007/s10552-009-9405-0 [DOI] [PubMed] [Google Scholar]
- 30.Stevens RG. Circadian disruption and breast cancer: from melatonin to clock genes. Epidemiology. 2005;16(2):254–258. [DOI] [PubMed] [Google Scholar]
- 31.Kos-Kudla B, Ostrowska Z, Kozlowski A, et al. Circadian rhythm of melatonin in patients with colorectal carcinoma. Neuro Endocrinol Lett. 2002;23(3):239–242. [PubMed] [Google Scholar]
- 32.Keith LG, Oleszczuk JJ, Laguens M. Circadian rhythm chaos: a new breast cancer marker. Int J Fertil Womens Med. 2001;46(5):238–247. [PubMed] [Google Scholar]
- 33.Gasche JA, Goel A. Epigenetic mechanisms in oral carcinogenesis. Future Oncol. 2012;8(11):1407–1425. doi: 10.2217/fon.12.138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masri S, Kinouchi K, Sassone-Corsi P. Circadian clocks, epigenetics, and cancer. Curr Opin Oncol. 2015;27(1):50–56. doi: 10.1097/CCO.0000000000000153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joska TM, Zaman R, Belden WJ. Regulated DNA methylation and the circadian clock: implications in cancer. Biology. 2014;3(3):560–577. doi: 10.3390/biology3030560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Straif K, Baan R, Grosse Y, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–1066. doi: 10.1016/S1470-2045(07)70373-X [DOI] [PubMed] [Google Scholar]
- 37.Kettner NM, Voicu H, Finegold MJ, et al. Circadian homeostasis of liver metabolism suppresses hepatocarcinogenesis. Cancer Cell. 2016;30(6):909–924. doi: 10.1016/j.ccell.2016.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dauchy RT, Xiang S, Mao L, et al. Circadian and melatonin disruption by exposure to light at night drives intrinsic resistance to tamoxifen therapy in breast cancer. Cancer Res. 2014;74(15):4099–4110. doi: 10.1158/0008-5472.CAN-13-3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papagiannakopoulos T, Bauer MR, Davidson SM, et al. Circadian rhythm disruption promotes lung tumorigenesis. Cell Metab. 2016;24(2):324–331. doi: 10.1016/j.cmet.2016.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duffy SA, Teknos T, Taylor JM, et al. Health behaviors predict higher interleukin-6 levels among patients newly diagnosed with head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22(3):374–381. doi: 10.1158/1055-9965.EPI-12-0987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duffield GE. DNA microarray analyses of circadian timing: the genomic basis of biological time. J Neuroendocrinol. 2003;15(10):991–1002. [DOI] [PubMed] [Google Scholar]
- 42.Qu Y, Wang Z, Huang X, et al. Circadian telomerase activity and DNA synthesis for timing peptide administration. Peptides. 2003;24(3):363–369. [DOI] [PubMed] [Google Scholar]
- 43.Koike N, Yoo SH, Huang HC, et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals. Science. 2012;338(6105):349–354. doi: 10.1126/science.1226339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mure LS, Le HD. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359:6381. doi: 10.1016/j.bbr.2018.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller BH, McDearmon EL, Panda S, et al. Circadian and CLOCK-controlled regulation of the mouse transcriptome and cell proliferation. Proc Natl Acad Sci U S A. 2007;104(9):3342–3347. doi: 10.1073/pnas.0611724104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunt T, Sassone-Corsi P. Riding tandem: circadian clocks and the cell cycle. Cell. 2007;129(3):461–464. [DOI] [PubMed] [Google Scholar]
- 47.Eismann EA, Lush E, Sephton SE. Circadian effects in cancer-relevant psychoneuroendocrine and immune pathways. Psychoneuroendocrinology. 2010;35(7):963–976. doi: 10.1016/j.psyneuen.2009.12.011 [DOI] [PubMed] [Google Scholar]
- 48.Huang XL, Fu CJ, Bu RF. Role of circadian clocks in the development and therapeutics of cancer. J Int Med Res. 2011;39(6):2061–2066. doi: 10.1177/147323001103900601 [DOI] [PubMed] [Google Scholar]
- 49.Sotak M, Sumova A, Pacha J. Cross-talk between the circadian clock and the cell cycle in cancer. Ann Med. 2014;46(4):221–232. doi: 10.3109/07853890.2014.892296 [DOI] [PubMed] [Google Scholar]
- 50.Bjarnason GA, Jordan RC, Wood PA, et al. Circadian expression of clock genes in human oral mucosa and skin: association with specific cell-cycle phases. Am J Pathol. 2001;158(5):1793–1801. doi: 10.1016/S0002-9440(10)64135-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matsuo T, Yamaguchi S, Mitsui S, Emi A, Shimoda F, Okamura H. Control mechanism of the circadian clock for timing of cell division in vivo. Science. 2003;302(5643):255–259. doi: 10.1126/science.1086271 [DOI] [PubMed] [Google Scholar]
- 52.Fu L, Pelicano H, Liu J, Huang P, Lee C. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell. 2002;111(1):41–50. [DOI] [PubMed] [Google Scholar]
- 53.Tan XM, Ye H, Yang K, et al. Circadian variations of clock gene Per2 and cell cycle genes in different stages of carcinogenesis in golden hamster buccal mucosa. Sci Rep. 2015;5:9997. doi: 10.1038/srep09997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borgs L, Beukelaers P, Vandenbosch R, Belachew S, Nguyen L, Cell MB. “circadian” cycle: new role for mammalian core clock genes. Cell Cycle. 2009;8(6):832–837. doi: 10.4161/cc.8.6.7869 [DOI] [PubMed] [Google Scholar]
- 55.Dang CV. MYC on the path to cancer. Cell. 2012;149(1):22–35. doi: 10.1016/j.cell.2012.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723 [DOI] [PubMed] [Google Scholar]
- 57.Grechez-Cassiau A, Rayet B, Guillaumond F, Teboul M, Delaunay F. The circadian clock component BMAL1 is a critical regulator of p21WAF1/CIP1 expression and hepatocyte proliferation. J Biol Chem. 2008;283(8):4535–4542. doi: 10.1074/jbc.M705576200 [DOI] [PubMed] [Google Scholar]
- 58.Yang X, Wood PA, Ansell CM, et al. The circadian clock gene Per1 suppresses cancer cell proliferation and tumor growth at specific times of day. Chronobiol Int. 2009;26(7):1323–1339. doi: 10.3109/07420520903431301 [DOI] [PubMed] [Google Scholar]
- 59.Gery S, Komatsu N, Baldjyan L, Yu A, Koo D, Koeffler HP. The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell. 2006;22(3):375–382. doi: 10.1016/j.molcel.2006.03.038 [DOI] [PubMed] [Google Scholar]
- 60.Gu X, Xing L, Shi G, et al. The circadian mutation PER2(S662G) is linked to cell cycle progression and tumorigenesis. Cell Death Differ. 2012;19(3):397–405. doi: 10.1038/cdd.2011.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sato F, Wu Y, Bhawal UK, et al. PERIOD1 (PER1) has anti-apoptotic effects, and PER3 has pro-apoptotic effects during cisplatin (CDDP) treatment in human gingival cancer CA9-22 cells. Eur J Cancer. 2011;47(11):1747–1758. doi: 10.1016/j.ejca.2011.02.025 [DOI] [PubMed] [Google Scholar]
- 62.Sato F, Nagata C, Liu Y, et al. PERIOD1 is an anti-apoptotic factor in human pancreatic and hepatic cancer cells. J Biochem. 2009;146(6):833–838. doi: 10.1093/jb/mvp126 [DOI] [PubMed] [Google Scholar]
- 63.Elshazley M, Sato M, Hase T, et al. The circadian clock gene BMAL1 is a novel therapeutic target for malignant pleural mesothelioma. Int J Cancer. 2012;131(12):2820–2831. doi: 10.1002/ijc.27598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Canaple L, Kakizawa T, Laudet V. The days and nights of cancer cells. Cancer Res. 2003;63(22):7545–7552. [PubMed] [Google Scholar]
- 65.Chan S, Rowbottom L, McDonald R, et al. Does the time of radiotherapy affect treatment outcomes? A review of the literature. Clin Oncol (R Coll Radiol). 2017;29(4):231–238. doi: 10.1016/j.clon.2016.12.005 [DOI] [PubMed] [Google Scholar]
- 66.Vincenzi B, Santini D, La Cesa A, Tonini G. Cancer chronotherapy: principles, applications, and perspectives. Cancer. 2003;98(4):881–882. author reply 882-883. doi: 10.1002/cncr.11600 [DOI] [PubMed] [Google Scholar]
- 67.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461(7267):1071–1078. doi: 10.1038/nature08467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28(5):739–745. doi: 10.1016/j.molcel.2007.11.015 [DOI] [PubMed] [Google Scholar]
- 69.Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science. 2012;336(6087):1440–1444. doi: 10.1126/science.1218351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fu XJ, Li HX, Yang K, Chen D, Tang H. The important tumor suppressor role of PER1 in regulating the cyclin-CDK-CKI network in SCC15 human oral squamous cell carcinoma cells. Onco Targets Ther. 2016;9:2237–2245. doi: 10.2147/OTT.S100952 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Li HX, Fu XJ, Yang K, Chen D, Tang H, Zhao Q. The clock gene PER1 suppresses expression of tumor-related genes in human oral squamous cell carcinoma. Oncotarget. 2016;7(15):20574–20583. doi: 10.18632/oncotarget.7827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang X, He X, Yang Z, Jabbari E. Mammalian PER2 regulates AKT activation and DNA damage response. Biochem Cell Biol. 2012;90(6):675–682. doi: 10.1139/o2012-025 [DOI] [PubMed] [Google Scholar]
- 73.Bouchard-Cannon P, Mendoza-Viveros L, Yuen A, Kaern M, Cheng HY. The circadian molecular clock regulates adult hippocampal neurogenesis by controlling the timing of cell-cycle entry and exit. Cell Rep. 2013;5(4):961–973. doi: 10.1016/j.celrep.2013.10.037 [DOI] [PubMed] [Google Scholar]
- 74.Gotoh T, Vila-Caballer M, Liu J, Schiffhauer S, Finkielstein CV. Association of the circadian factor Period 2 to p53 influences p53‘s function in DNA-damage signaling. Mol Biol Cell. 2015;26(2):359–372. doi: 10.1091/mbc.E14-05-0994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Unsal-Kacmaz K, Chastain PD, Qu PP, et al. The human Tim/Tipin complex coordinates an Intra-S checkpoint response to UV that slows replication fork displacement. Mol Cell Biol. 2007;27(8):3131–3142. doi: 10.1128/MCB.02190-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang X, Wood PA, Hrushesky WJ. Mammalian TIMELESS is required for ATM-dependent CHK2 activation and G2/M checkpoint control. J Biol Chem. 2010;285(5):3030–3034. doi: 10.1074/jbc.M109.050237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Engelen E, Janssens RC, Yagita K, Smits VA, van der Horst GT, Tamanini F. Mammalian TIMELESS is involved in period determination and DNA damage-dependent phase advancing of the circadian clock. PLoS One. 2013;8(2):e56623. doi: 10.1371/journal.pone.0056623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papp SJ, Huber AL, Jordan SD, et al. DNA damage shifts circadian clock time via Hausp-dependent Cry1 stabilization. Elife. 2015;4. doi:10.7554/eLife.04883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim J, D‘Annibale S, Magliozzi R, et al. USP17- and SCFbetaTrCP–regulated degradation of DEC1 controls the DNA damage response. Mol Cell Biol. 2014;34(22):4177–4185. doi: 10.1128/MCB.00530-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang F, Li C, Yongluo CL. The circadian gene clock plays an important role in cell apoptosis and the DNA damage response in vitro. Technol Cancer Res Treat. 2016;15(3):480–486. doi: 10.1177/1533034615585433 [DOI] [PubMed] [Google Scholar]
- 81.Cotta-Ramusino C, McDonald ER 3rd, Hurov K, Sowa ME, Harper JW, Elledge SJ. A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. Science. 2011;332(6035):1313–1317. doi: 10.1126/science.1203430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mullenders J, Fabius AW, Madiredjo M, Bernards R, Beijersbergen RL. A large scale shRNA barcode screen identifies the circadian clock component ARNTL as putative regulator of the p53 tumor suppressor pathway. PLoS One. 2009;4(3):e4798. doi: 10.1371/journal.pone.0004798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13(3):332–337. [DOI] [PubMed] [Google Scholar]
- 84.Miki T, Matsumoto T, Zhao Z, Lee CC. p53 regulates Period2 expression and the circadian clock. Nat Commun. 2013;4:2444. doi: 10.1038/ncomms3444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–310. doi: 10.1038/35042675 [DOI] [PubMed] [Google Scholar]
- 86.Xiong H, Yang Y, Yang K, Zhao D, Tang H, Ran X. Loss of the clock gene PER2 is associated with cancer development and altered expression of important tumor-related genes in oral cancer. Int J Oncol. 2018;52(1):279–287. doi: 10.3892/ijo.2017.4180 [DOI] [PubMed] [Google Scholar]
- 87.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82(4):675–684. [DOI] [PubMed] [Google Scholar]
- 88.el-Deiry WS, Tokino T, Velculescu VE, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75(4):817–825. [DOI] [PubMed] [Google Scholar]
- 89.Hua H, Wang Y, Wan C, et al. Circadian gene mPer2 overexpression induces cancer cell apoptosis. Cancer Sci. 2006;97(7):589–596. doi: 10.1111/j.1349-7006.2006.00225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sun CM, Huang SF, Zeng JM, et al. Per2 inhibits k562 leukemia cell growth in vitro and in vivo through cell cycle arrest and apoptosis induction. Pathol Oncol Res. 2010;16(3):403–411. doi: 10.1007/s12253-009-9227-0 [DOI] [PubMed] [Google Scholar]
- 91.Gotoh T, Vila-Caballer M, Santos CS, Liu J, Yang J, Finkielstein CV. The circadian factor Period 2 modulates p53 stability and transcriptional activity in unstressed cells. Mol Biol Cell. 2014;25(19):3081–3093. doi: 10.1091/mbc.E14-05-0993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee S, Donehower LA, Herron AJ, Moore DD, Fu L. Disrupting circadian homeostasis of sympathetic signaling promotes tumor development in mice. PLoS One. 2010;5(6):e10995. doi: 10.1371/journal.pone.0010995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu J, Zou X. Distinct control of PERIOD2 degradation and circadian rhythms by the oncoprotein and ubiquitin ligase MDM2. Sci Signal. 2018;11(556). [DOI] [PubMed] [Google Scholar]
- 94.Wang Y, Solt LA, Kojetin DJ, Burris TP. Regulation of p53 stability and apoptosis by a ROR agonist. PLoS One. 2012;7(4):e34921. doi: 10.1371/journal.pone.0034921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Altman BJ, Hsieh AL, Sengupta A, et al. MYC disrupts the circadian clock and metabolism in cancer cells. Cell Metab. 2015;22(6):1009–1019. doi: 10.1016/j.cmet.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walz S, Lorenzin F, Morton J, et al. Activation and repression by oncogenic MYC shape tumour-specific gene expression profiles. Nature. 2014;511(7510):483–487. doi: 10.1038/nature13473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–1133. doi: 10.1038/ng.2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boone DN, Qi Y, Li Z, Hann SR. Egr1 mediates p53-independent c-Myc-induced apoptosis via a noncanonical ARF-dependent transcriptional mechanism. Proc Natl Acad Sci U S A. 2011;108(2):632–637. doi: 10.1073/pnas.1008848108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang C, Tai Y, Lisanti MP, Liao DJ. c-Myc induction of programmed cell death may contribute to carcinogenesis: a perspective inspired by several concepts of chemical carcinogenesis. Cancer Biol Ther. 2011;11(7):615–626. doi: 10.4161/cbt.11.7.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Soucek L, Whitfield JR, Sodir NM, et al. Inhibition of Myc family proteins eradicates KRas-driven lung cancer in mice. Genes Dev. 2013;27(5):504–513. doi: 10.1101/gad.205542.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Felsher DW. MYC inactivation elicits oncogene addiction through both tumor cell-intrinsic and host-dependent mechanisms. Genes Cancer. 2010;1(6):597–604. doi: 10.1177/1947601910377798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lin CY, Loven J, Rahl PB, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell. 2012;151(1):56–67. doi: 10.1016/j.cell.2012.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sabo A, Kress TR, Pelizzola M, et al. Selective transcriptional regulation by Myc in cellular growth control and lymphomagenesis. Nature. 2014;511(7510):488–492. doi: 10.1038/nature13537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolf E, Lin CY, Eilers M, Levens DL. Taming of the beast: shaping Myc-dependent amplification. Trends Cell Biol. 2015;25(4):241–248. doi: 10.1016/j.tcb.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kress TR, Sabo A, Amati B. MYC: connecting selective transcriptional control to global RNA production. Nat Rev Cancer. 2015;15(10):593–607. doi: 10.1038/nrc3984 [DOI] [PubMed] [Google Scholar]
- 106.Lorenzin F, Benary U, Baluapuri A, et al. Different promoter affinities account for specificity in MYC-dependent gene regulation. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huber AL, Papp SJ, Chan AB, et al. CRY2 and FBXL3 Cooperatively Degrade c-MYC. Mol Cell. 2016;64(4):774–789. doi: 10.1016/j.molcel.2016.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Repouskou A, Sourlingas TG, Sekeri-Pataryas KE, Prombona A. The circadian expression of c-MYC is modulated by the histone deacetylase inhibitor trichostatin A in synchronized murine neuroblastoma cells. Chronobiol Int. 2010;27(4):722–741. doi: 10.3109/07420521003786800 [DOI] [PubMed] [Google Scholar]
- 109.Shostak A, Ruppert B, Ha N, et al. MYC/MIZ1-dependent gene repression inversely coordinates the circadian clock with cell cycle and proliferation. Nat Commun. 2016;7:11807. doi: 10.1038/ncomms11807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Repouskou A, Prombona A. c-MYC targets the central oscillator gene Per1 and is regulated by the circadian clock at the post-transcriptional level. Biochim Biophys Acta. 2016;1859(4):541–552. doi: 10.1016/j.bbagrm.2016.02.001 [DOI] [PubMed] [Google Scholar]
- 111.Granda TG, Liu XH, Smaaland R, et al. Circadian regulation of cell cycle and apoptosis proteins in mouse bone marrow and tumor. FASEB J. 2005;19(2):304–306. doi: 10.1096/fj.04-2665fje [DOI] [PubMed] [Google Scholar]
- 112.Koyanagi S, Kuramoto Y, Nakagawa H, et al. A molecular mechanism regulating circadian expression of vascular endothelial growth factor in tumor cells. Cancer Res. 2003;63(21):7277–7283. [PubMed] [Google Scholar]
- 113.Falcao AS, Kataoka MS, Ribeiro NA, et al. A novel cell line derived from pleomorphic adenoma expresses MMP2, MMP9, TIMP1, TIMP2, and shows numeric chromosomal anomalies. PLoS One. 2014;9(8):e105231. doi: 10.1371/journal.pone.0105231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ye H, Yang K, Tan XM, Fu XJ, Li HX. Daily rhythm variations of the clock gene PER1 and cancer-related genes during various stages of carcinogenesis in a golden hamster model of buccal mucosa carcinoma. Onco Targets Ther. 2015;8:1419–1426. doi: 10.2147/OTT.S83710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bjarnason GA, Jordan RC, Sothern RB. Circadian variation in the expression of cell-cycle proteins in human oral epithelium. Am J Pathol. 1999;154(2):613–622. doi: 10.1016/S0002-9440(10)65306-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anea CB, Ali MI, Osmond JM, et al. Matrix metalloproteinase 2 and 9 dysfunction underlie vascular stiffness in circadian clock mutant mice. Arterioscler Thromb Vasc Biol. 2010;30(12):2535–2543. doi: 10.1161/ATVBAHA.110.214379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zieker D, Jenne I, Koenigsrainer I, et al. Circadian expression of clock- and tumor suppressor genes in human oral mucosa. Cell Physiol Biochem. 2010;26(2):155–166. doi: 10.1159/000320547 [DOI] [PubMed] [Google Scholar]
- 118.Jensen LD, Cao Z, Nakamura M, et al. Opposing effects of circadian clock genes bmal1 and period2 in regulation of VEGF-dependent angiogenesis in developing zebrafish. Cell Rep. 2012;2(2):231–241. doi: 10.1016/j.celrep.2012.07.005 [DOI] [PubMed] [Google Scholar]
- 119.Oda A, Katayose Y, Yabuuchi S, et al. Clock gene mouse period2 overexpression inhibits growth of human pancreatic cancer cells and has synergistic effect with cisplatin. Anticancer Res. 2009;29(4):1201–1209. [PubMed] [Google Scholar]
- 120.Zhanfeng N, Yanhui L, Zhou F, Shaocai H, Guangxing L, Hechun X. Circadian genes Per1 and Per2 increase radiosensitivity of glioma in vivo. Oncotarget. 2015;6(12):9951–9958. doi: 10.18632/oncotarget.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wu Y, Sato F, Uk B, et al. Basic helix-loop-helix transcription factors DEC1 and DEC2 regulate the paclitaxel-induced apoptotic pathway of MCF-7 human breast cancer cells. Int J Mol Med. 2011;27(4):491–495. doi: 10.3892/ijmm.2011.617 [DOI] [PubMed] [Google Scholar]
- 122.Liu Y, Sato F, Kawamoto T, et al. Anti-apoptotic effect of the basic helix-loop-helix (bHLH) transcription factor DEC2 in human breast cancer cells. Genes Cells. 2010;15(4):315–325. doi: 10.1111/j.1365-2443.2010.01381.x [DOI] [PubMed] [Google Scholar]
- 123.Jung CH, Kim EM, Park JK, et al. Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol Rep. 2013;29(6):2109–2113. doi: 10.3892/or.2013.2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Matsumoto CS, Almeida LO, Guimaraes DM, et al. PI3K-PTEN dysregulation leads to mTOR-driven upregulation of the core clock gene BMAL1 in normal and malignant epithelial cells. Oncotarget. 2016;7(27):42393–42407. doi: 10.18632/oncotarget.9877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–192. doi: 10.1038/nrc1819 [DOI] [PubMed] [Google Scholar]
- 126.Lee JH, Sancar A. Regulation of apoptosis by the circadian clock through NF-kappaB signaling. Proc Natl Acad Sci U S A. 2011;108(29):12036–12041. doi: 10.1073/pnas.1108125108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jensen LD, Cao Y. Clock controls angiogenesis. Cell Cycle. 2013;12(3):405–408. doi: 10.4161/cc.23596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sato F, Uk B, Kawamoto T, et al. Basic-helix-loop-helix (bHLH) transcription factor DEC2 negatively regulates vascular endothelial growth factor expression. Genes Cells. 2008;13(2):131–144. doi: 10.1111/j.1365-2443.2007.01153.x [DOI] [PubMed] [Google Scholar]
- 129.Hsu CM, Lin SF, Lu CT, Lin PM, Yang MY. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012;33(1):149–155. doi: 10.1007/s13277-011-0258-2 [DOI] [PubMed] [Google Scholar]
- 130.Cao Q, Zhao X, Bai J, et al. Circadian clock cryptochrome proteins regulate autoimmunity. Proc Natl Acad Sci U S A. 2017;114(47):12548–12553. doi: 10.1073/pnas.1619119114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cermakian N, Lange T, Golombek D, et al. Crosstalk between the circadian clock circuitry and the immune system. Chronobiol Int. 2013;30(7):870–888. doi: 10.3109/07420528.2013.782315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibbs JE, Blaikley J, Beesley S, et al. The nuclear receptor REV-ERBalpha mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(2):582–587. doi: 10.1073/pnas.1106750109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349(1):82–90. doi: 10.1016/j.mce.2011.06.039 [DOI] [PubMed] [Google Scholar]
- 134.Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25(4):2761–2765. [PubMed] [Google Scholar]
- 135.Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain Behav Immun. 2003;17(5):321–328. [DOI] [PubMed] [Google Scholar]
- 136.Curtis AM, Bellet MM, Sassone-Corsi P, O‘Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40(2):178–186. doi: 10.1016/j.immuni.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 137.Taniguchi H, Fernandez AF, Setien F, et al. Epigenetic inactivation of the circadian clock gene BMAL1 in hematologic malignancies. Cancer Res. 2009;69(21):8447–8454. doi: 10.1158/0008-5472.CAN-09-0551 [DOI] [PubMed] [Google Scholar]
- 138.Sun Y, Yang Z, Niu Z, et al. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology. 2006;119(4):451–460. doi: 10.1111/j.1365-2567.2006.02456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spengler ML, Kuropatwinski KK, Comas M, et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc Natl Acad Sci U S A. 2012;109(37):E2457–E2465. doi: 10.1073/pnas.1206274109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Arjona A, Sarkar DK. The circadian gene mPer2 regulates the daily rhythm of IFN-gamma. J Interferon Cytokine Res. 2006;26(9):645–649. doi: 10.1089/jir.2006.26.645 [DOI] [PubMed] [Google Scholar]
- 141.Yan J, Wang H, Liu Y, Shao C. Analysis of gene regulatory networks in the mammalian circadian rhythm. PLoS Comput Biol. 2008;4(10):e1000193. doi: 10.1371/journal.pcbi.1000193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Narasimamurthy R, Hatori M, Nayak SK, Liu F, Panda S, Verma IM. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc Natl Acad Sci U S A. 2012;109(31):12662–12667. doi: 10.1073/pnas.1209965109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhou M, Wang W, Karapetyan S, et al. Redox rhythm reinforces the circadian clock to gate immune response. Nature. 2015;523(7561):472–476. doi: 10.1038/nature14449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tong X, Buelow K, Guha A, Rausch R, Yin L. USP2a protein deubiquitinates and stabilizes the circadian protein CRY1 in response to inflammatory signals. J Biol Chem. 2012;287(30):25280–25291. doi: 10.1074/jbc.M112.340786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sand L, Wallstrom M, Hirsch JM. Smokeless tobacco, viruses and oral cancer. Oral Health Dent Manag. 2014;13(2):372–378. [PubMed] [Google Scholar]
- 146.Yuan H, Li H, Ma H, et al. Genetic polymorphisms in key DNA repair genes and risk of head and neck cancer in a Chinese population. Exp Ther Med. 2012;3(4):719–724. doi: 10.3892/etm.2012.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Amit M, Yen TC, Liao CT, et al. Improvement in survival of patients with oral cavity squamous cell carcinoma: an international collaborative study. Cancer. 2013;119(24):4242–4248. doi: 10.1002/cncr.28357 [DOI] [PubMed] [Google Scholar]
- 148.Wolff D, Hassfeld S, Hofele C. Influence of marginal and segmental mandibular resection on the survival rate in patients with squamous cell carcinoma of the inferior parts of the oral cavity. J Craniomaxillofac Surg. 2004;32(5):318–323. doi: 10.1016/j.jcms.2004.05.005 [DOI] [PubMed] [Google Scholar]
- 149.Johnson NW, Warnakulasuriya S, Gupta PC, et al. Global oral health inequalities in incidence and outcomes for oral cancer: causes and solutions. Adv Dent Res. 2011;23(2):237–246. doi: 10.1177/0022034511402082 [DOI] [PubMed] [Google Scholar]
- 150.Zhao N, Tang H, Yang K, Chen D. Circadian rhythm characteristics of oral squamous cell carcinoma growth in an orthotopic xenograft model. Onco Targets Ther. 2013;6:41–46. doi: 10.2147/OTT.S39955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hsu CM, Lin PM, Lai CC, Lin HC, Lin SF, Yang MY. PER1 and CLOCK: potential circulating biomarkers for head and neck squamous cell carcinoma. Head Neck. 2014;36(7):1018–1026. doi: 10.1002/hed.23402 [DOI] [PubMed] [Google Scholar]
- 152.Chen R, Yang K, Zhao NB, et al. Abnormal expression of PER1 circadian-clock gene in oral squamous cell carcinoma. Onco Targets Ther. 2012;5:403–407. doi: 10.2147/OTT.S38508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Su X, Chen D, Yang K, et al. The circadian clock gene PER2 plays an important role in tumor suppression through regulating tumor-associated genes in human oral squamous cell carcinoma. Oncol Rep. 2017;38(1):472–480. doi: 10.3892/or.2017.5653 [DOI] [PubMed] [Google Scholar]
- 154.Tang Q, Cheng B, Xie M, et al. Circadian clock gene Bmal1 inhibits tumorigenesis and increases paclitaxel sensitivity in tongue squamous cell carcinoma. Cancer Res. 2017;77(2):532–544. doi: 10.1158/0008-5472.CAN-16-1322 [DOI] [PubMed] [Google Scholar]