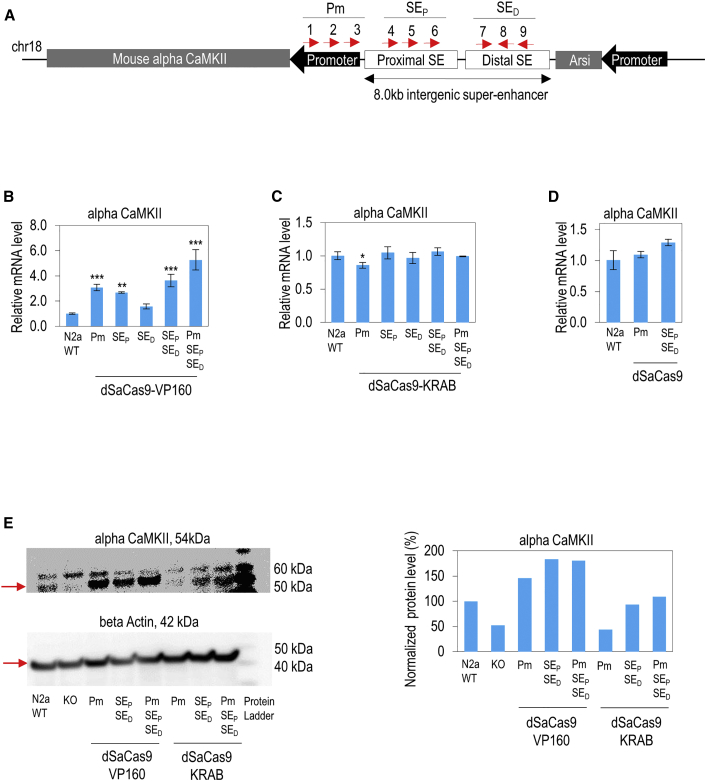
Figure 3.
Modulation of Endogenous Alpha CaMKII Expressions by Using Minimal CRISPRa and CRISPRi Transgenes Targeting Super-Enhancer and Promoter in Mouse N2a Cells
(A) sgRNA target sites on super-enhancer and promoter of mouse alpha CaMKII. The locations of nine different sgRNA target sites designed to target the promoter (Pm), proximal super-enhancer (SEP), and distal super-enhancer (SED) are shown. Red arrows indicate the sense or antisense orientation of sgRNAs designed to recognize target DNA sequences. (B) dSaCas9-VP160-mediated activation of endogenous alpha CaMKII mRNA level in mouse N2a cells. Nine dSaCas9-VP160 activators were designed to target the Pm, SEP, and SED. Expression levels of alpha CaMKII in N2a cells are shown. (C) dSaCas9-KRAB-mediated repression of endogenous alpha CaMKII mRNA level in mouse N2a cells. Nine dSaCas9-KRAB repressors were designed to target the Pm, SEP, and SED. Expression levels of alpha CaMKII in N2a cells are shown. (D) dSaCas9-mediated transcriptional interference of mouse alpha CaMKII. (E) Western blot for protein lysate of alpha CaMKII in mouse N2a cells. The monoclonal mouse alpha CaMKII- (54 kDa) specific antibody was used. Beta-actin (Actb, 42 kDa) was used as an internal control. Protein expression levels of alpha CaMKII were measured for wild-type (WT), knockout (KO), and under the effects of dSaCas9-VP160 and dSaCas9-KRAB in N2a cells. Right panel shows the normalized protein expression level of alpha CaMKII in N2a cells. (B-D) The statistical significance levels from control (wild-type N2a) are indicated as *p < 0.05, **p < 0.01, and ***p < 0.001. All data are presented as mean ± SD (n = 3).