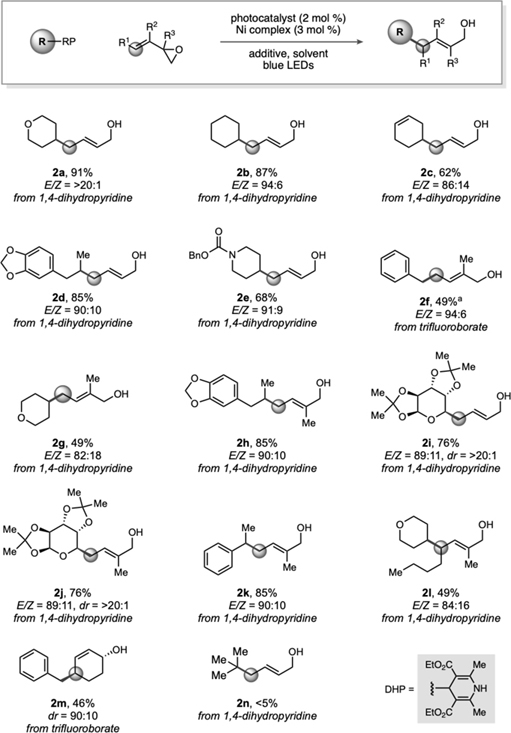
Table 2.
Reactions were conducted on 0.50 mmol scale. Standard reaction conditions employed butadiene monoxide (1.0 equiv), 1,4-dihydropyridine (1.5 equiv), 4CzIPN (2 mol %), and [Ni(dtbbpy)(H2O)4]Cl2 (3 mol %) in acetone. For trifluoroborates, standard reaction conditions employed butadiene monoxide (1.0 equiv), alkyltrifluoroborate (1.5 equiv), Ir[dFCF3ppy]2(bpy)PF6 (2 mol %), [Ni(dtbbpy)(H2O)4]Cl2 (3 mol %), and 2,6-lutidine (3.5 equiv) in acetone/MeOH (9:1). For α-alkoxyalkyltrifluoroborates, a solvent mixture of acetonitrile/tert-butanol (10:1) was used.
 |
