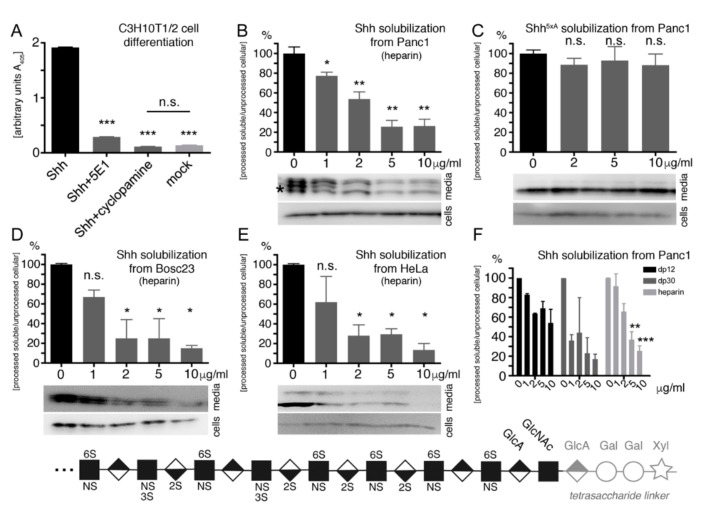
Figure 3.
Heparin-modulated Shh release. (A) Shh expressed in Panc1 cells is bioactive, as indicated by Hh-dependent C3H10T1/2 reporter cell differentiation into alkaline phosphatase-producing osteoblasts. Shh biofunction was specifically inhibited by 5E1 and cyclopamine. *** denotes statistical significance (p < 0.0001, n = 2). (B) Increasing amounts of soluble heparin reduced Shh processing from Panc1 cells in a dose-dependent manner. * and ** denote statistical significance (p < 0.05 and p < 0.005, respectively, n = 5–8). (C) Impaired Shh release was specifically due to a blockade of the CW processing site because the release of Shh5xA lacking all HS-binding basic amino acids remained unaffected. n.s.: not significant (p > 0.05, n = 5–10). Shh release from Bosc23 cells (D) and HeLa cells (E) was also inhibited by increasing heparin concentrations. (F) Small heparin oligosaccharides (dp12 and dp30) variably reduce Shh release from Panc1 cells. Bottom: heparin structure. Most acetyl groups from GlcNAc residues are replaced by sulfate groups to generate an extended N-sulfated (NS) domain. Extensive subsequent modifications, such as epimerization of GlcA to IdoA and 2-O, 6-O, and 3-O sulfations generate a highly sulfated, negatively charged region. Xyl: xylose, Gal: galactose, GlcA: glucuronic acid, GlcNAc: N-acetylglucosamine, IdoA: iduronic acid. Modified after [69,70].