fig. 1. Ric-8A is constitutively phosphorylated in cells.
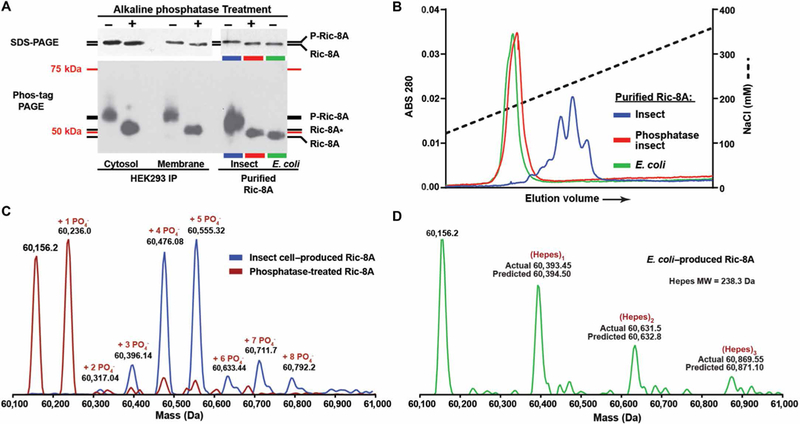
(A) Gel mobility shift assays of endogenous Ric-8A immunoprecipitated (IP) from cytosolic and detergent-extracted membrane fractions (5× material loaded) of HEK293 cells, and recombinant Ric-8A purified from E. coli or insect cells before and after alkaline phosphatase treatment. Top: Standard SDS-PAGE. Bottom: Phos-tag PAGE. Ric-8A* may have phosphosite(s) partially resistant to alkaline phosphatase. The molecular mass markers (75 and 50 kDa) do not accurately reflect the true masses of Ric-8A proteins on the Phos-tag PAGE. Data are representative of more than three independent experiments. (B) Anion exchange chromatography resolution of recombinant Ric-8A purified from E. coli or from insect cells before and after alkaline phosphatase treatment. Data are representative of more than three independent experiments. (C) Mass spectra of Ric-8A proteins were obtained through whole-protein ESI/MS analysis. Spectra are of insect cell–purified recombinant rat Ric-8A (blue trace) and alkaline phosphatase–treated Ric-8A (red trace). (D) The ESI-MS/MS spectrum of E. coli–purified Ric-8A revealed a completely unmodified protein (mass, 60156.2 Da) with a series of Hepes buffer adducts. MW, molecular weight.
