fig. 3. Gαi1 binds phosphorylated Ric-8A with a higher affinity than it has for unphosphorylated Ric-8A.
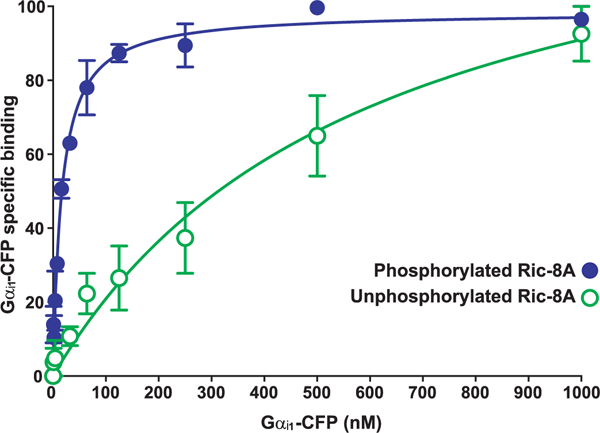
A concentration series of purified Gαi1CFP (1 to 1000 nM) was incubated with insect cell– or E. coli–produced GST-tagged Ric-8A or GST to determine the nonspecific component. Glutathione-coated microspheres were used to adsorb the GST-Ric-8A-Gαi1-CFP complexes before flow cytometric measurement of bead-associated fluorescence. Data were gated to include only singlet beads and then were analyzed for median fluorescence intensity (MFI). Nonspecific binding (GST only) was subtracted from total to yield the percentage of Gαi1-CFP bound specifically to Ric-8A, with 100% indicating the maximum fluorescence of saturated binding. The plotted data are the result of three independent experiments, and error bars indicate the SEM. The data were fitted to one-site specific binding curves with GraphPad Prism software.
