Fig. 4. Requirements of the Thr440 and Ser435 phosphosites for Ric-8A-stimulated Gαq steady-state GTP hydrolysis activity.
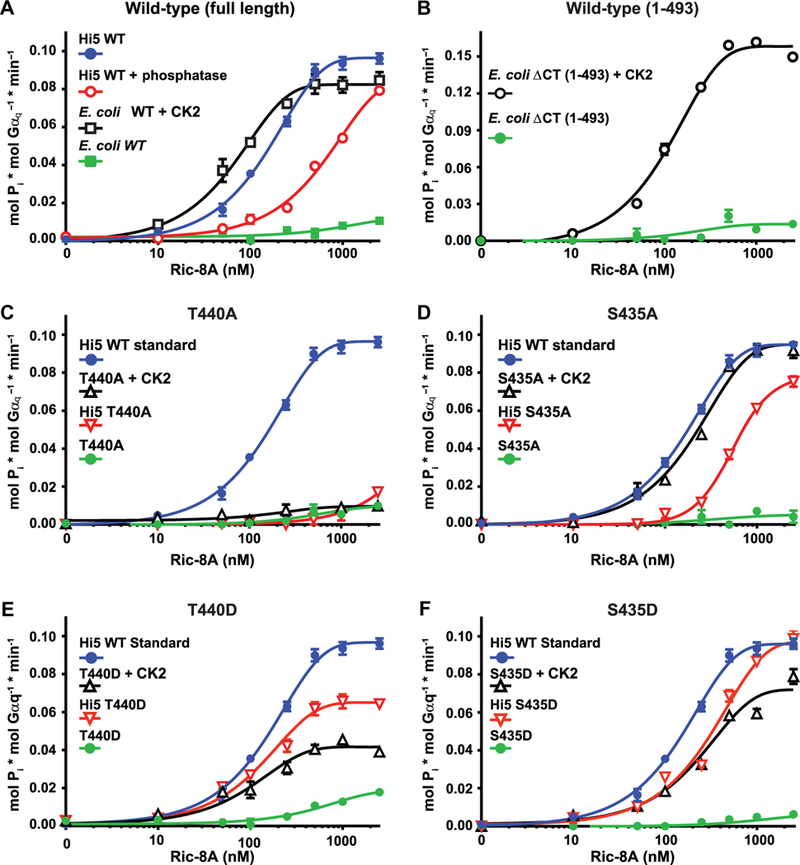
(A to F) Purified Gαq (50 nM) was incubated with the indicated concentrations of purified Ric-8A proteins and [ γ−32P]GTP. The linear rate of GTP hydrolysis was determined by measuring the production of free 32Pi. (A) Full-length WT Ric-8A proteins purified from insect cells (Hi5) and treated with or without alkaline phosphatase or purified from E. coli and treated with or without CK2 were tested for their ability to stimulate the steady-state GTPase activity of Gαq. (B) WT Ric-8A-ACT protein purified from E. coli and treated with or without CK2 was tested for its ability to stimulate the steady-state GTPase activity of Gαq. (C to F) WT Ric-8A purified from insect cells (Hi5) was used as a positive standard of phosphorylated Ric-8A activity for comparison to the activities of (C) Ric-8A–T440A purified from insect cells and Ric-8A–T440A purified from E. coli and treated with or without CK2, (D) Ric-8A–S435A purified from insect cells and Ric-8A-S435A purified from E. coli and treated with or without CK2, (E) Ric-8A-T440D purified from insect cells and Ric-8A-T440D purified from E coli and treated with or without CK2, and (F) Ric-8A-S435D purified from insect cells and Ric-8A-S435D purified from E. coli and treated with or without CK2. Data were plotted on semilog graphs and fitted to one-phase exponential association functions using GraphPad Prism. Experiments were performed in triplicate. Error bars indicate the SEM and were sometimes smaller than the size of the plotted symbols.
