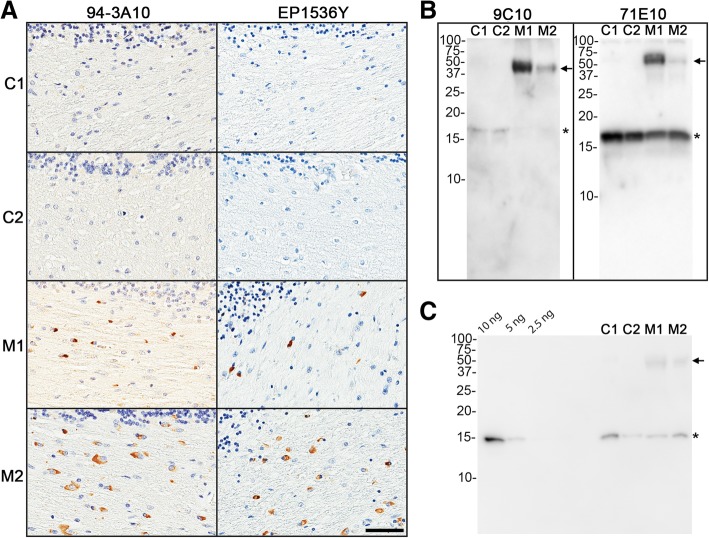
Fig. 1.
Immunohistochemical and biochemical determination of aggregated αS inclusion pathology in the white matter cerebellum of MSA and control patients used to generate lysates as inoculum. The cerebellum of control (C1 and C2) and MSA (M1 and M2) cases utilized for lysate injections were screened for the absence or presence, respectively, of aggregated pathological αS by immunohistochemistry and western blot analysis. a Immunohistochemistry was performed with anti-αS antibody 94-3A10 and anti-pSer129 αS antibody EP1536Y. Scale bar = 50 μm. b Immunoblotting was performed with anti-αS antibodies 9C10 and 71E10, where 9C10, preferentially reacts with aggregated αS. c Immunoblotting of known amounts (10, 5 and 2.5 ng) of recombinant human αS protein to determine the relative amount of αS within experimental lysates. 2 μL of each sample, as identified above the lanes, was loaded and probed with human αS specific antibody 33A-3F3. For the western blots, the mobility of molecular mass markers are indicted on the left. The band corresponding to monomeric αS is indicated by an asterisks and arrows indicate higher molecular, aggregated αS species. For quantification this analysis was repeated