Figure 1.
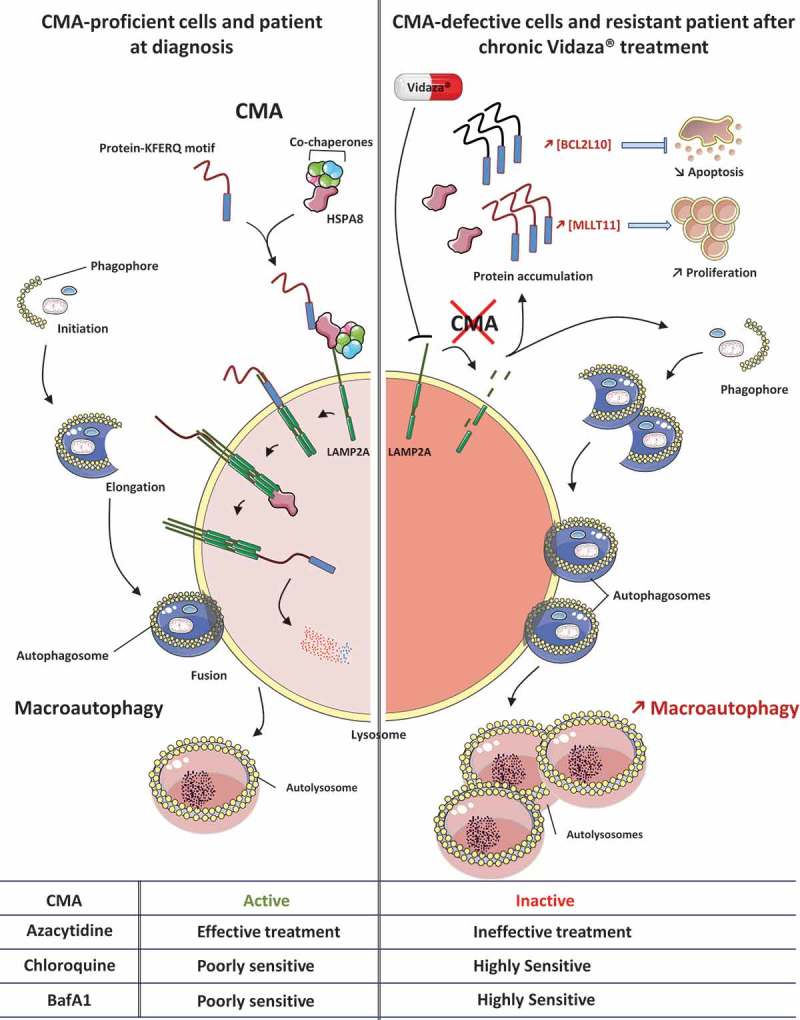
Inhibition of CMA is compensated by activation of macroautophagy in Aza-resistant MDS and AML cell lines and patients. In CMA-proficient cells and MDS-AML patients at diagnosis, CMA is fully operational and protein substrates endowed with a KFERQ-like motif are readily degraded within lysosomes. Macroautophagy also proceeds efficiently and allows the elimination of unfolded proteins and damaged organelles to ensure proper cellular homeostasis. In CMA-defective cells or MDS-AML patients chronically treated with Aza, progressive loss of LAMP2 expression through epigenetic mechanisms leads to CMA defects and accumulation of CMA substrates. Among them, BCL2L10 and MLLT11/AF1Q exert crucial roles in cell fate, limiting apoptosis on the one hand and increasing proliferation on the other hand to promote cell survival and potentially leukemogenesis. This allows the emergence of LAMP2-negative cells with higher proliferative capacities. In Aza-resistant MDS and AML cells, the defect of CMA is compensated by an increase in macroautophagy, that can be therapeutically exploited through the use of lysosomal inhibitors, and more specially FDA-approved molecules such as hydroxychloroquine. BafA1, bafilomycin A1.
