Fig. 3.
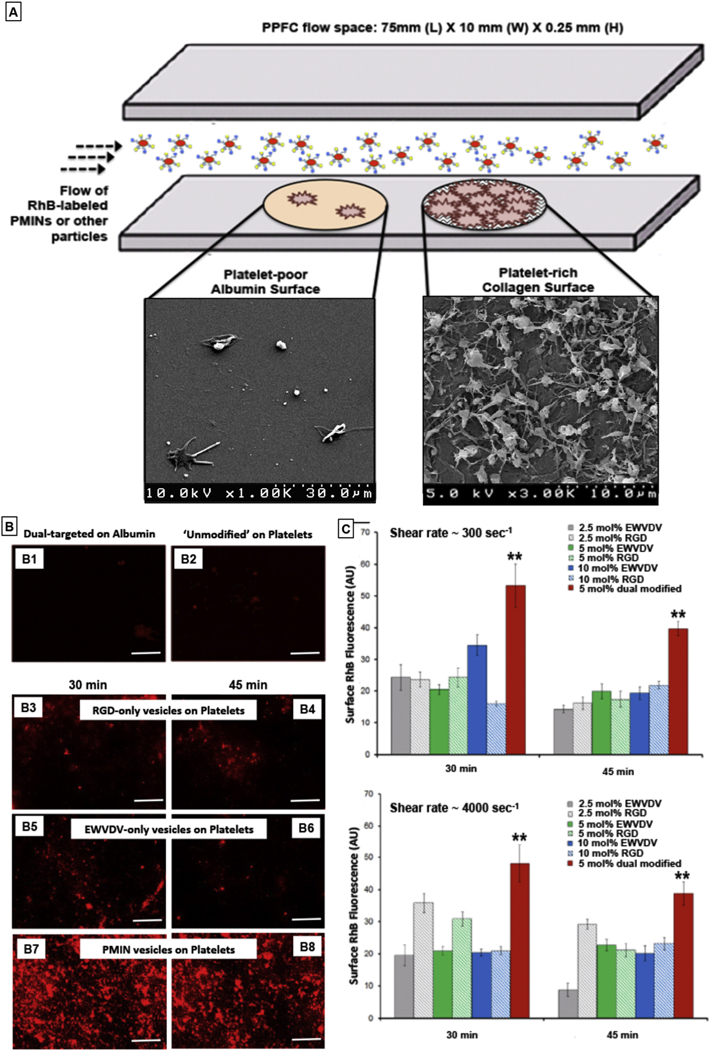
[A] Experimental set-up schematic of parallel plate flow chamber (PPFC) where platelet-poor albumin-coated regions (control surface) and active platelet-rich thrombus region (on collagen-coated area) were created and unmodified RhB-labeled (red fluorescent) unmodified vesicles or singly modified vesicles (bearing RGD decorations or EWVDV decorations only) or dual modified PMINs (bearing both peptide decorations) were flowed over these surfaces at various flow rates (low-to-high shear rates); [B] Representative fluorescent images of particle binding shows that (B1) dual-targeted particles (i.e. PMINs) have minimal binding on albumin-coated surface and (B2) unmodified particles have minimal binding on the platelet-rich thrombus surface; (B3, B4) RGD-decorated vesicles and (B5, B6) EWVDV-decorated vesicles have reasonable extent of binding and retention on the platelet-rich thrombus surface, but the level of binding and retention levels are significantly enhanced for (B7, B8) dual modified PMINs; [C] Quantitative analysis of vesicle binding and retention (based on surface-averaged RhB fluorescence intensity) from multiple batches of experiments show that irrespective of flow conditions (low or high shear rate), dual modified vesicles (PMINs) have significantly higher binding and retention capabilities compared to singly modified vesicles even when the mol% composition of single peptide modification is to twice (10 mol%) that of dual peptide modification (5 mol%).
