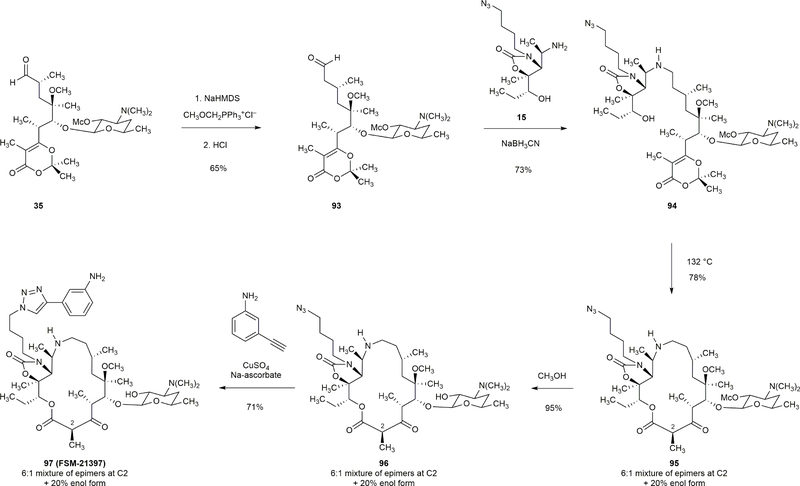
Extended Data Figure 7 |. Synthesis of a 16-membered azaketolide.
Homologation of aldehyde 35 was achieved by a Wittig olefination reaction (CH3OCH3PPh3+Cl–, NaHMDS) followed by hydrolysis of the resulting enol ether to afford aldehyde 93 in 65% yield. Reductive coupling of amine 15 and aldehyde 93 furnished macrocyclization precursor 94 (73% yield). The 16-membered macrolactone 95 was obtained in 78% yield upon thermolysis of 94 (1mM, 132 °C). Two additional steps transformed 95 to the 16-membered azaketolide FSM-21397.