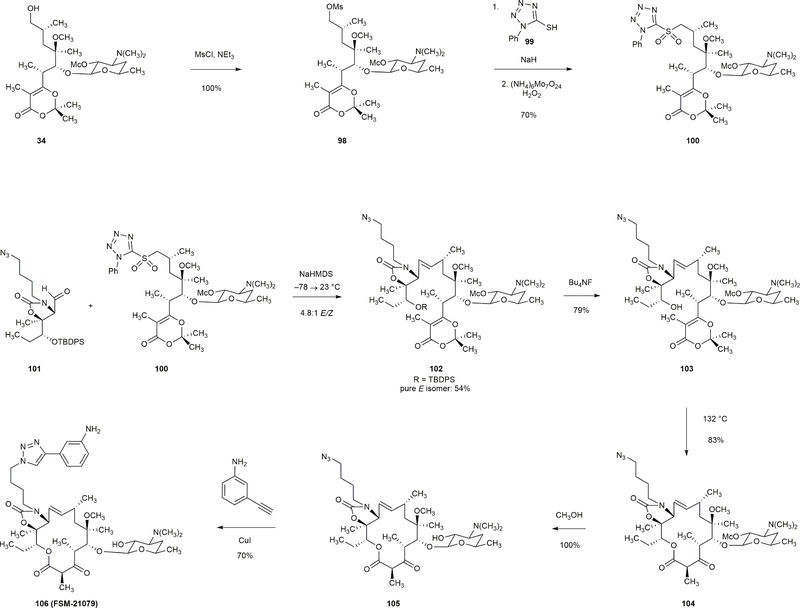
Extended Data Figure 8 |. Synthesis of a 14-membered macrolide with a trans-olefin linkage.
Mesylate 98 was prepared in quantitative yield by treatment of alcohol 34 with methanesulfonyl chloride (1.50 equiv) and triethylamine (2.0 equiv). Displacement of mesylate 98 with sodium 1-phenyl-1H-tetrazole-5-thiolate (2.0 equiv) followed by oxidation of the resulting thioether with ammonium molybdate (0.20 equiv)–hydrogen peroxide (100 equiv) afforded sulfone 100 in 70% yield. Aldehyde 101 and sulfone 100 were coupled in a Julia–Kocienski olefination reaction (NaHMDS, –78 → 23 °C) to provide a 4.8:1 mixture of E- and Z-olefin isomers. The E-isomer 102 was isolated and desilylated (Bu4NF, 79%). Thermolysis of the product 103 (1 mM, 132 °C) furnished the 14-membered macrocycle 104 in 83% yield. 104 was then transformed to FSM-21079 in two additional steps (methanolysis and [3+2] dipolar cycloaddition).