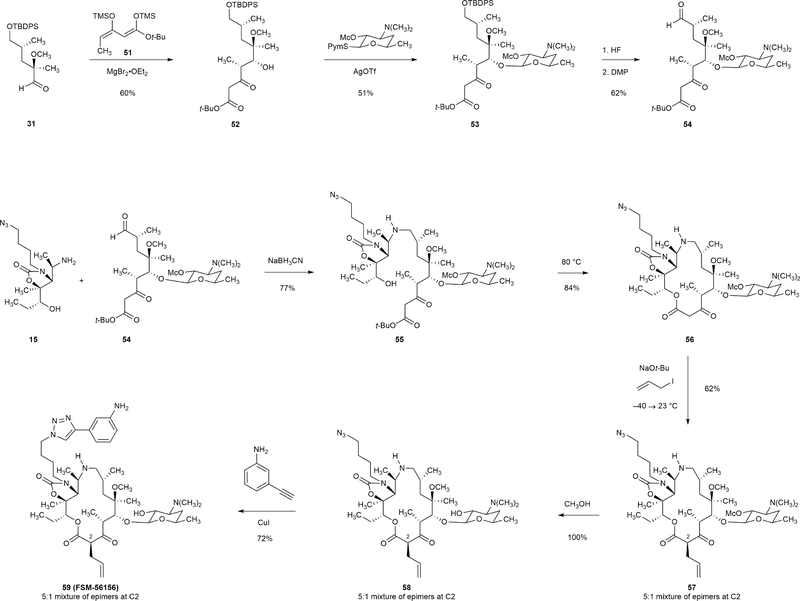
Extended Data Figure 2 |. Synthesis of a 15-membered azaketolide with a modified C2-substituent.
Thermolysis of a β-keto tert-butyl ester substrate (55) proceeded at a lower temperature (80 °C) and afforded a 15-membered macrocycle without substitution at C2 (56). This macrocycle served as a nearly ideal intermediate for preparation of macrolides with diverse C2-substitutions. For example, an allyl group was introduced at C2 by treatment of 56 with sodium tert-butoxide (1.1 equiv) and allyl iodide (1.1 equiv) at –40 °C followed by warming the reaction solution to 23 °C. The product 57 (obtained in 62% yield) was then transformed to FSM-56156 in two steps (72% yield).