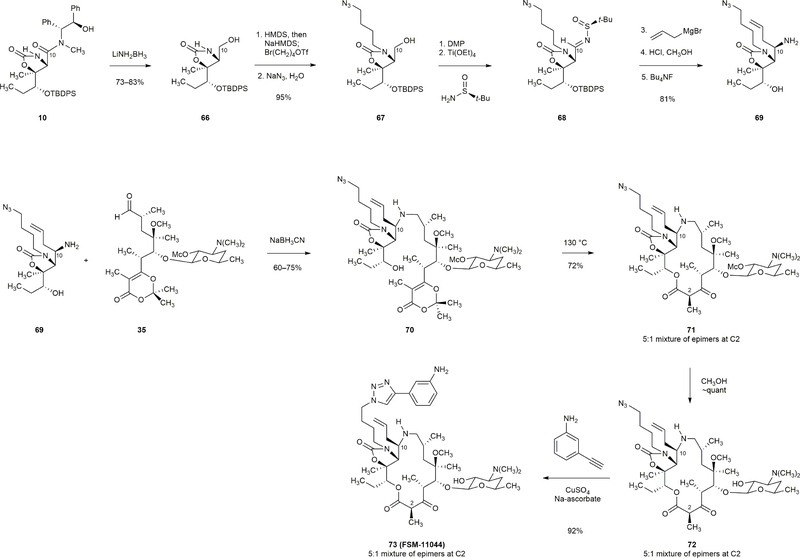
Extended Data Figure 4 |. Synthesis of a 15-membered azaketolide with a modified C10-substituent.
N-tert-butylsulfinyl imine 68 (prepared in five steps from amide 10) allowed for the stereocontrolled introduction of various C10-substituents. For example, addition of allylmagnesium bromide proceeded with >20:1 stereoselectively to furnish the adduct depicted; subsequent cleavage of the sulfinyl (HCl, CH3OH) and tert-butyldiphenylsilyl (Bu4NF) groups within the adduct then furnished left-hand intermediate 69 (81% yield). Amine 69 and aldehyde 35 were coupled by a reductive amination reaction (NaBH3CN, 60–75% yield). The product (70) was then transformed to FSM-11044 in a three-step sequence that consisted of a macrocyclization reaction (72% yield), a methanolysis reaction (quantitative yield) and lastly a [3+2] dipolar cycloaddition reaction (92% yield).