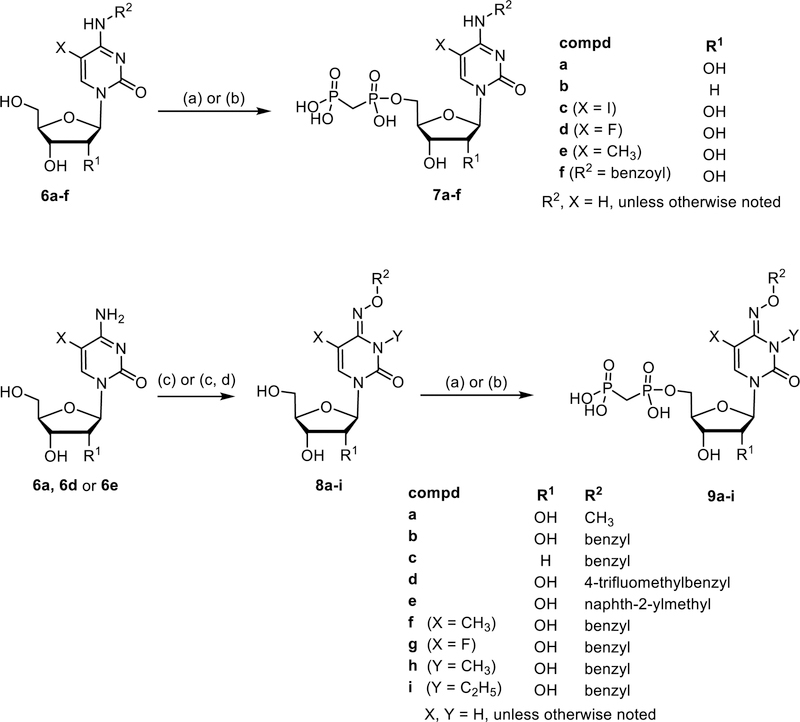
Scheme 1.
Synthesis of adenosine 2a–g and uridine derivatives 4a–y. Reagents and conditions: (a) DCC (3 eq.), methylene diphosphonic acid (1.5 eq.), DMF, room temp, 3–24 h; for compounds 4w and 4x: DCC (3 eq.), ethylene diphosphonic acid (1.5 eq.), DMF, room temp, 3 h (b) methylenebis(phosphonic dichloride) (3 eq.), trimethyl phosphate, 0 °C, 30 min, then triethylammonium hydrogencarbonate buffer pH 8.4–8.6, rt, 30 min.