Table 2.
The inhibitory potency of uridine-derived nucleotides 4a-y as rat CD73 inhibitors. R2, X, Y = H, W = O, V = CH, Z = N, n = 1, unless otherwise noted.
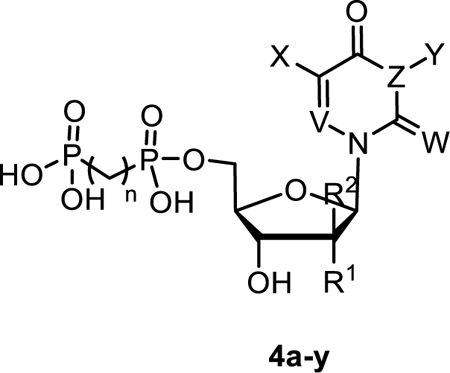 | ||||
|---|---|---|---|---|
| Compd. | Substitution | R1 | R2 | Ki ± SEM (nM) (% inhibition at indicated concentration), rat CD73 |
| UOPCP 4a | OH | H | 1830 ± 530 | |
| 4b | Y = CH3 | OH | H | 1860 ± 400 |
| 4c | Y = C2H5 | OH | H | > 1000 (4%) |
| 4d | Y = C3H7 | OH | H | > 1000 (7%) |
| 4e | Y = benzyl | OH | H | > 1000 (3%) |
| 4f | X = CH3 | OH | H | 338 ± 56 |
| 4g | X = CH3 | H | H | 639 ± 65 |
| 4h | X = CH3 | OCH3 | H | > 1000 (3%) |
| 4i | X = ethynyl | OH | H | 276 ± 37 |
| 4j | X = 1-chlorovinyl | OH | H | 424 ± 27 |
| 4k | X = 1-chlorovinyl, Y = CH3 | OH | H | 1050 ± 290 |
| 4l | X = F | OH | H | 14.8 ± 1.9 |
| 4m | X = Cl | OH | H | 86.7 ± 7.6 |
| 4n | X = Br | OH | H | 88.7 ± 12.5 |
| 4o | X = I | OH | H | 162 ± 4 |
| 4p | H | H | > 1000 (37%) | |
| 4q | NH2 | H | > 1000 (2%) | |
| 4r | N3 | H | > 1000 (9%) | |
| 4s | F | H | > 1000 (11%) | |
| 4t | H | F | 1750± 380 | |
| 4u | H | OH | > 1000 (8%) | |
| 4v | V = N | OH | H | > 1000 (21%) |
| 4w | n = 2 | OH | H | > 1000 (4%) |
| 4x | n= 2, X = CH3 | OH | H | > 1000 (9%) |
| 4y | W = S | OH | H | > 1000 (7%) |
