ABSTRACT
Acute pancreatitis (AP) is a digestive disease characterized by pancreatic inflammation. Tetramethylpyrazine (TMP) has been effectively used to ameliorate the damage on intestinal mucosa injury in rats with acute necrotizing pancreatitis (ANP). We aim to study the protective effect of TMP on caerulein-induced AP and to explore the possible mechanism. The mice randomized into control and different experimental groups. AP was induced in mice by 6-hourly intraperitoneal (i.p) injections of caerulein (50 μg/kg at 1 h interval). TMP (i.p, 10 mg/kg, 1 h interval) was administered 3 h before caerulein injection. Administration of TMP attenuated the severity of AP as shown by the histopathology, reduced serum amylase activity and pro-inflammatory cytokines TNF-α and IL-6. Further, TMP enhances the beneficial effect by reducing caerulein-induced NF-κB activation and inducing cell apoptosis in pancreas. Therefore, inhibition of nuclear factor-kappa B(NF-κB) signals by TMP represents a potential therapeutic strategy for the treatment of acute pancreatitis.
KEYWORDS: Tetramethylpyrazine, nuclear factor-kappa B, apoptosis, cytokines, acute pancreatitis
Graphical Abstract
Introduction
Acute pancreatitis (AP) is caused by toxins that induce acinar cell calcium overload, zymogen activation, cytokine release and cell death [1]. Over the last two decades, our understanding of pathogenesis has advanced, but there is still no specific therapy despite many randomized trials [2]. Specific therapy for AP is lacking and deciphering the molecular mechanisms underlying its pathogenesis will likely aid in therapeutic intervention. The transcription factor NF-κB was as a nuclear factor that binds to the enhancer element of the immunoglobulin kappa light-chain of activated B cells (thereby coining the abbreviation NF-κB) and plays a variety of evolutionarily conserved roles in the immune system. The NF-κB is activated early in acinar cells during acute pancreatitis and increases expression of multiple proinflammatory genes, increasing vascular permeability and inducing thrombosis and hemorrhage, ultimately leading to tissue necrosis [3,4]. Therefore, reducing NF-κB activation and proinflammatory mediators might be a good therapeutic strategy to attenuate AP.
Apoptosis and necrosis are two major forms of acinar cell death in AP and associated with specific morphological and biochemical features [5,6]. Pancreatic acinar cell death occurs principally via apoptosis or necrosis. Although the consequences of apoptosis and necrosis are distinct in pancreatitis, the mechanisms underlying these two types of cell death are interrelated and they both involve mitochondria [5,7]. Apoptosis, by contrast to necrosis, does not elicit an inflammatory response and hence does not injure the surrounding cells, having an active protective effect [7–9], suggesting that enhancing apoptosis could reduce the severity of AP [7,10]. A large body of evidence has demonstrated a protective role of NF-κB in most tissues and cell types. For example, targeted deletion of p65 in mice leads to increased apoptosis in several tissues [11]. The protection by NF-κB is due to transcriptional activation of a number of antiapoptotic proteins, such as Bcl-2 and Bcl-XL [12,13].Therefore, targeting NF-κB signaling pathway could result in improved prognoses through increased apoptosis in AP.
Tetramethylpyrazine (TMP) is one of the major active constituents of the traditional Chinese herbal medicine, Ligusticum wallichii Franchat (chuanxiong). TMP has angiogenesis and vessel protection effect as well as anti-inflammatory function. It has been used to treat cardiovascular and inflammatory diseases clinically in China for a long time [14]. It has recently found that TMP can ameliorate the damage to pancreas and intestine caused by AP [15,16]. However, the exact mechanism underlying the effect of TMP in AP has not been completely addressed. Multiple studies have shown that TMP inhibits NF-kB activity, thereby causing inhibition of inflammatory factor release and protecting cells from damage [17–19]. In addition, TMP significantly induced apoptosis and G0/G1 arrest in osteosarcoma cells by downregulating the protein expression of nuclear NF-κB p65(P65), bcl-2 and cyclin D1 [20].
In the present study, we evaluate the therapeutic effectiveness and mechanisms of TMP on AP in rat models. Our findings show that TMP promotes apoptosis and inhibits necrosis, decreases the pancreatic inflammatory response and ameliorates acute pancreatitis in mice through inhibition of NF-κB activation, nuclear p65 translocation and bcl-2 expression.
Materials and methods
Ethics statement
The experimental protocol was approved by the institutional animal ethics committee of the central hospital of Linyi, with animal care performed strictly according to the guide for the care and use of laboratory animals published by the US National Institutes of Health (NIH Publication No.85–23, revised 1996). All surgery was performed under pentobarbital anesthesia, and all efforts were made to minimize suffering.
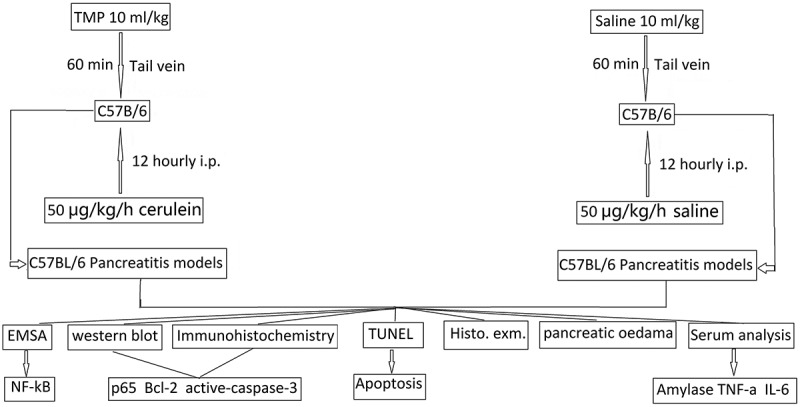
Pancreatitis models
All animal experiments were approved by the Animal Ethics Committee of Linyi central hospital and carried out in accordance with the International Guiding Principles for Animal Research. For induction of pancreatitis, age – and sex-matched C57BL/6 were fasted for 18 h but provided with water and libitum (10 animals per experimental group). Mice received 6-hourly i.p. injections of saline (control) or 50 μg/kg/h cerulein (Sigma-Aldrich, St. Louis, MO, USA) in saline. Animals were killed by CO2 asphyxiation 1 h after the final caerulein injection. The blood and tissue samples were collected for studies. To assess the severity of cerulein-induced pancreatitis, H&E-stained tissue sections from all experimental groups were graded by two blinded independent observers, using the method described previously [22].
TMP administration
TMP (purity >98%) was purchased from Sigma. The dosages of TMP were chosen based on previous reports [23,24]. TMP was administered via i.p in a volume of 10 ml/kg for 1 h starting 3 h before the administration of cerulein for induction of pancreatitis and control animals were given i.p injection of saline for 1 h.
Electrophoretic mobility shift assay (EMSA)
Electrophoresis Mobility Shift Assay (EMSA) was performed as reported previously [25]. Briefly, nuclear and cytoplasmic extraction reagents were used to extract the nuclear proteins of tissues. The BCA method was used to measure protein concentration. The NF-κB probe AGT TGA GGG GAC TTT CCC AGG C (Santa Cruz Biotechnology, Shanghai, China) was labeled with [α-32P] dCTP, which were incubated with 10 µg nuclear extracts for 30 min at room temperature. Anti-p65 antibody (BD Pharmingen) was used to observe a supershift. The reaction mixture was electrophoresed on 4% polyacrylamide gels, and the gel with separated samples was dried and subjected to autoradiography using phosphor screens at −80°C.
Western blotting
Pancreas tissues were lysed in 20 mM Tris-HCl, pH 8.0, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 5 mM NaF, 50 mM 2-glycerophosphate, and protease inhibitors (Roche, Shanghai, China). Then, the lysates above were centrifuged at 12,000 rpm at 4°C for 10 min. Samples were separated through an SDS-PAGE, transferred to Immobilon P membranes, and western blotting was performed with specific antibodies against p65 (Santa Cruz Biotechnology), cleaved-caspase-3, bcl-2 and, as a loading control, an anti-GAPDH antibody (Sigma). Appropriate fluorescent dye-labeled secondary antibodies were used to allow detection with the Odyssey Infrared Imaging System (LI-COR Biosciences) as previously described [26]. Blots are representative of at least three experiments.
Immunohistochemistry (IHC)
Immunohistochemistry was performed in accordance with the instructions of the SP-9001 Kit (Beijing Nobleryder Technology Co. Ltd., Beijing, China). The paraffin-embedded pancreatic tissue blocks obtained from the mice of the normal and AP groups were placed at room temperature for 30 min. The tissues were then fixed with acetone at 4°C for 10 min, dewaxed, rehydrated, exhaust the endogenous peroxidase activity, incubated with rabbit anti-NF-Kbp65,bcl-2 antibody (Abcam Inc.,Cambridge, MA, USA) at 4°C overnight, then incubated with a corresponding biotinylated goat anti-rabbit IgG secondary antibody as the previously described[27]. The samples were dehydrated with graded ethanol, permeabilized with xylene and mounted by neutral balsam. Phosphate-buffered saline (PBS) was regarded as the control during the replacement of the primary antibody. The experiment was repeated 3 times. The scores of staining intensity and cell rate of positive expression were calculated using the OlymPusDp70 image acquisition analyzer. The scale of staining intensity was as follows: 0, no staining; 1, weak staining; 2, moderate staining; 3, strong staining. The criteria for the cell rate of positive expression were as follows: 0, <1%; 1, <10%; 2, <50%; 3, <80%; 4, >80%; The final score was calculated based on staining intensity and cell rate of positive expression: 0–2, negative (-); 3–5, positive (+); 6–7, strongly positive (++).
TUNEL assay
For detection of cell death, the TUNEL (Terminal deoxynucleotidyl-transferase mediated dUTP Nick End Labelling) method was performed as previously described [28] and according to the Apop-Tag Plus kit (Chemicon Internacional, Shanghai,China). Sections adhered to silanized slides (3-aminopropyltrithoxysylane – Sigma-Aldrich Chemical Co., St. Louis, USA) were treated with 20 μg/ml proteinase K (Sigma- Aldrich Chemical Co., St. Louis, USA) and immersed in 3% hydrogen peroxide. After immersion in equilibration buffer for 20 min, the sections were incubated in TdT enzyme (Terminal deoxynucleotidyl Transferase) at 37°C for 1 h in a humidified chamber. The reaction was stopped by immersion in a stop/wash buffer for 20 min and incubated in anti-digoxigenin-peroxidase in a humidified chamber at 37°C for 30 min. The reaction was revealed with 0.06% 3.3‘-diaminobenzidine tetrahydrochloride (DAB-Sigma-Aldrich Chemical Co., Hangzhou, China) and counterstained with Carazzi’s hematoxylin. Sections of involuting mammary gland, provided by the manufacturer of the Kit, were used as positive controls for the TUNEL method. The sections used as negative controls were submitted to the same protocol, except the step of incubation in the TdT enzyme. Total apoptotic cells were calculated by counting the number of TUNEL-positive cells.
Histological examination
For light microscopy, fresh specimens of murine pancreas were fixed in 4% paraformaldehyde in PBS (pH 7.4). The tissues were embedded in paraffin, and 5 mm sections were processed for hematoxylin and eosin (H&E) staining by standard procedures. Multiple randomly chosen microscopic fields from at least three mice in each group were examined and scored by two pathologists in a blind manner based on the presence of vacuolization, interstitial edema, interstitial inflammation, the number of acinar cell necroses, as previously described [29]. The scoring assessment was performed on a scale of 0–3 (0 being normal and 3 being severe) on each parameter mentioned above and the sum of the scores were used to evaluate the severity of acute pancreatitis.
Quantification of pancreatic oedema
Pancreatic edema was estimated as water content. After exsanguination of the rat, a portion of the pancreas about 1 g in wet weight was excised and weighed. The tissue was dried in a vacuum centrifuge for 36 h at 60°C and reweighed. The resulting dry weights were subtracted from the original wet weights, yielding an estimate of the water content of the pancreata. The difference between wet weight and dry weight was calculated. The increased water content of the tissue was expressed as a percentage of the water content of a normal rat pancreas.
Serum amylase analysis
The extent of the pancreatic injury was determined by measuring serum amylase level using Phadebas reagent (Magle Life Sciences) as recommended by the supplier.
Measurement of TNF-α and IL-6
The serum levels of TNF-α and IL-6 were determined using enzyme-linked immunosorbent assay (ELISA) kits (EIAab, Shanghai, China) as the manufacturer's instruction.
Statistical analysis
Results were expressed as mean ± standard error of the mean from multiple separate experiments. Statistical analysis was performed using t-tests with SPSS version 16 software (IBM, Portsmouth, UK). Statistical significance was set at P values <0.05.
Results
TMP protected from caerulein-induced pancreatitis in mice
Mice were sacrificed 1 h after the final injection of caerulein. From inspection of H&E-stained sections, it was clear that acute pancreatic damage had been induced by the caerulein in pancreata of mice. Caerulein-injection led to a pronounced pancreatic edema, hemorrhage, leucocyte infiltration and necrosis (Figure1(a)). However, each of these features was markedly reduced by the prior administration of TMP (Figure1(a)). Furthermore, administration of TMP significantly decreased histological scoring and necrosis compared with the cerulein alone treated mice (Figure1(b,c)).
Figure 1.
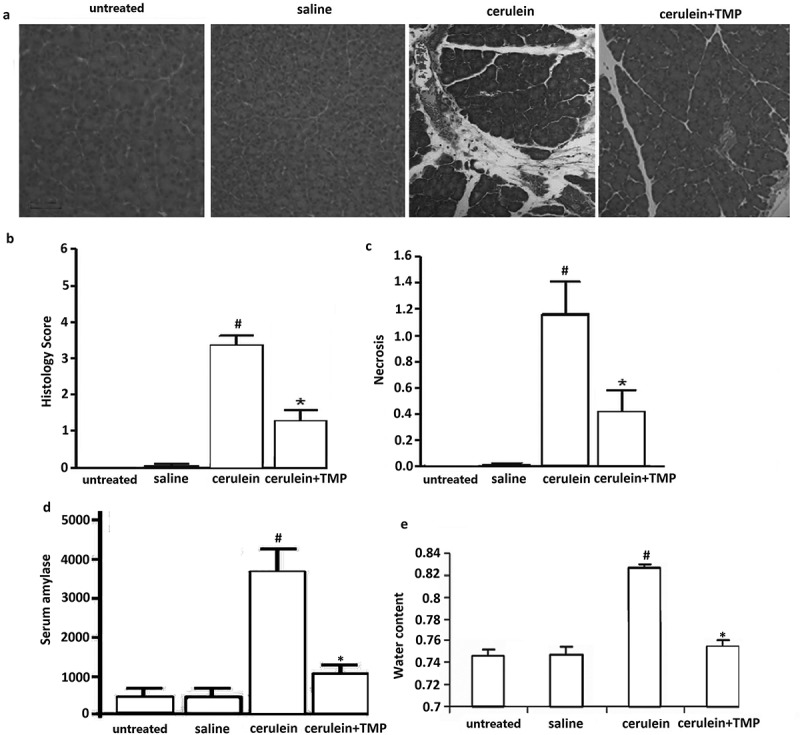
Evaluation of cerulein-treated and TMP-treated pancreatic tissue damage in mice. (a), Pathological morphology of pancreatic tissues in each group measured by haematoxylin-eosin (HE) staining. (b), Representative H&E images of pancreas histology slides from treatment groups; (c), Necrotic cells were counted on H&E-stained pancreas sections as percentage of total cells. D, Effect of TMP on serum amylase activity (U/L) of cerulien-induced acute pancreatitis in mice (i.p.). (e), Effect of TMP on pancreatic water content in the cerulein treated mice. vs untreated and saline,#p< 0.05; vs cerulein,*p< 0.05.
The evidence of pancreatic injury induced by caerulein is generally confirmed by an increase in plasma amylase. Thus, the effect of TMP treatment on plasma amylase in AP was evaluated, especially in comparison with the corresponding values in saline pretreated mice. As shown in Figure1(d), mice treated with caerulein injections, pancreatitis was manifested by a significant rise in serum amylase activity compared to the mice injected with hourly saline only (P< 0.05). However, among the TMP treatment groups, serum amylase was significantly reduced in mice (P< 0.05).
To determine the percentage of pancreatic water in the various groups, tissues were dried for 36 h at 90°C. The results showed that caerulein injections alone significantly increased relative water content in the pancreata. In contrast, the TMP treated pancreata exhibited only slight increases in the relative water content following caerulein injections (Figure 1(e), p < 0.05).
DHA reduced serum TNF-α and IL-6 in the cerulein-induced mice
We examined serum IL-6 and TNF-αlevels, and the results showed a significantly enhanced level of IL-6 and TNF-α in the cerulein-induced pancreatitic models, and this was significantly reduced in TMP-treated mice (Figure 2(a)).These data demonstrate that TMP inhibits serum TNF-α and IL-6 levels in cerulein-induced AP in mice.
Figure 2.
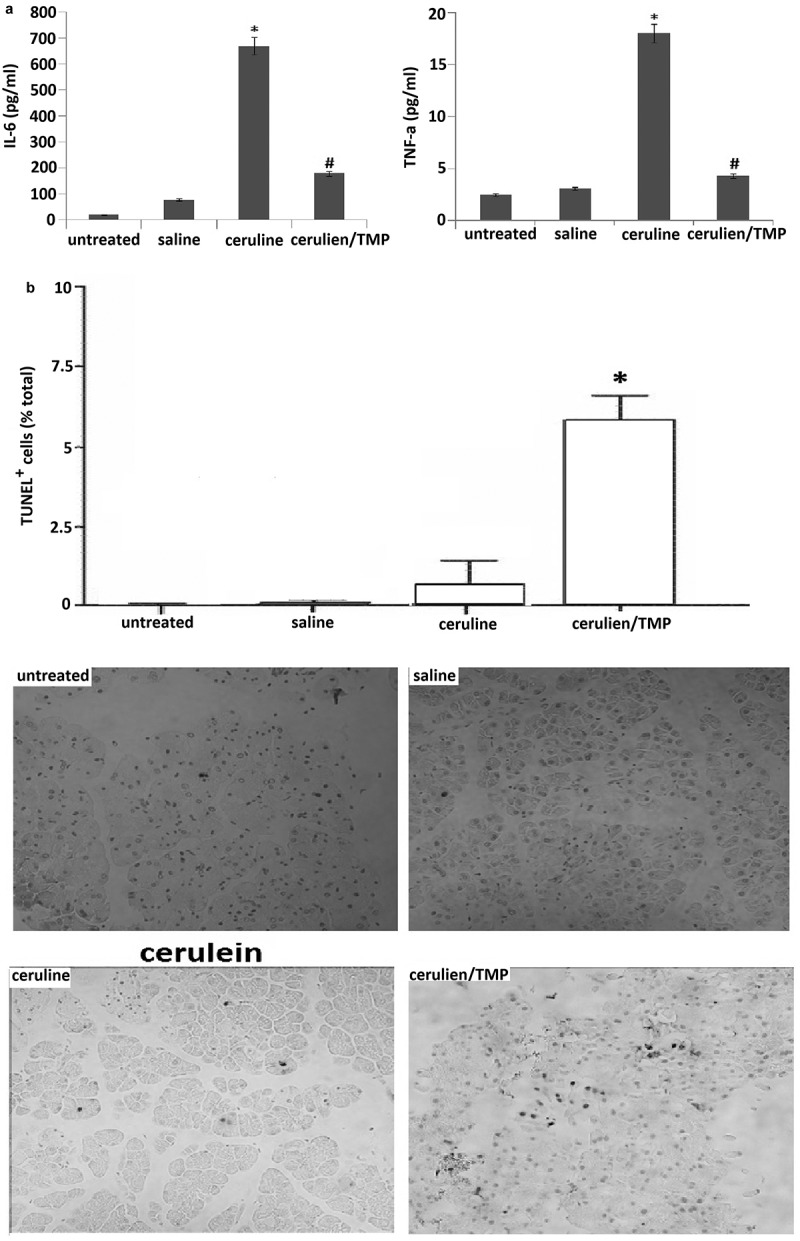
TMP inhibits serum TNF-α and IL-6 levels and promotes apoptosis in cerulein-induced pancreatitis. (a), Serum levels of interleukin (IL)-6 and tumor necrosis factor (TNF)-αwere detected 12 h after induction of acute pancreatitis or treated with TMP before the induction of acute pancreatitis. (b), Cell apoptosis was detected by TUNEL assay. Data are presented as mean ± SEM.*P< 0.05; #P< 0.05.
TMP induces apoptosis in cerulein-induced pancreatitis
We have analyzed apoptosis using TUNEL assay on pancreatic tissue sections (Figure 2(b)). Quantification for these assay data confirmed that there were more apoptotic cells in the pancreatic tissue of TMP-treated mice (5.6 ± 1.2) than that of in the cerulein alone treated mice (1.2 ± 0.3) (P< 0.05). These results demonstrate the TMP treatment induces an apoptotic response in pancreatic tissue.
TMP inhibited cerulein-induced activation of NF-kB in the pancreas
Here, we investigated the effect of TMP on cerulein-induced activation of NF-κB by determining the NF-κB-DNA binding activity in the pancreas. As shown in Figure 3(a), NF-κB was activated in the pancreas of cerulein-induced acute pancreatitis. However, TMP suppressed the cerulein-induced activation of NF-κB in the pancreas. In addition, a significant increase in nuclear transcription of p65 in the pancreas of cerulein-induced acute pancreatitis by Western blot assay (Figure 3(b)) and immunohistochemistry assay (Figure 3(c)). However, nuclear transcription of p65 was significantly inhibited in TMP-treated pancreas (Figure 3(a–c)).
Figure 3.
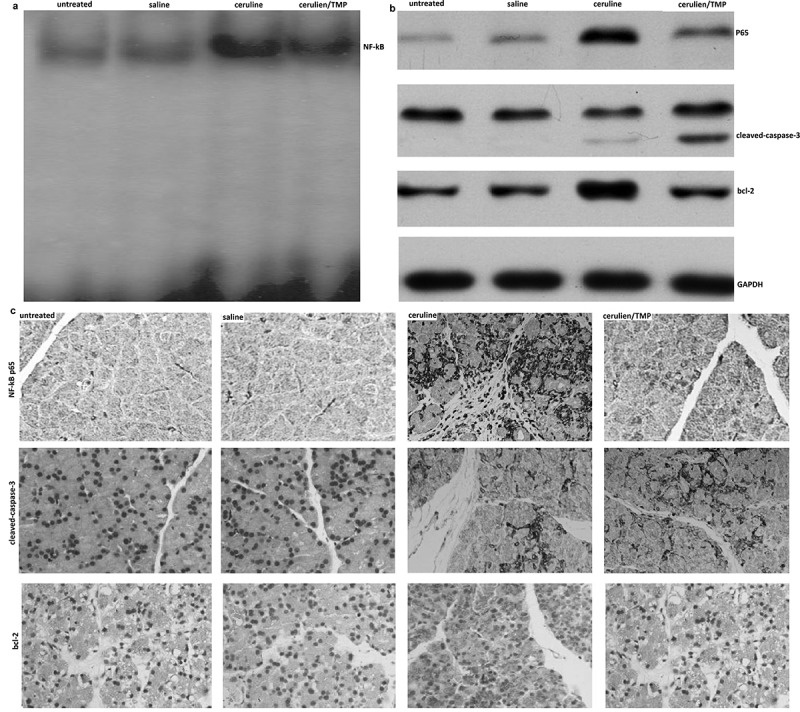
Changes in pancreatic levels of NF-Kb P65 nuclear transcription, Bcl-2 protein expression and caspase-3 activation in models of acute pancreatitis. (a), EMSA assay; (b), western blot assay; (c), Immunohistochemistry (IHC).
Western blot analysis showed that the prosurvival proteins Bcl-2 were present in normal rat pancreas, and were up-regulated in models of acute pancreatitis (Figure 3(b-c)). Up-regulation of pancreatic Bcl-2 protein was detected in all models examined, namely pancreatitis induced by cerulein in mice. However, the levels of bcl-2 were inhibited by TMP (Figure 3(b-c)). In addition, caspase-3 was activated in TMP treated pancreas, indicating that TMP increased cell apoptosis in mice pancreas (Figure3 (b-c)).
Discussion
The current therapeutic guidelines for acute pancreatitis (AP) include the following: intravenous fluid replacement, dietary changes, analgesics, inhibitors of pancreatic secretion (somatostatin and its analog, octreotide), L-arginine, calcium ion antagonists, and different inflammatory mediator inhibitors [30–33]. Unfortunately, the use of standard drugs in acute pancreatitis is still disappointing.
Chinese herbal formulas have upheld the holistic therapeutic philosophy for thousands of years, which consists of two or more appropriate medicinal plants or animals according to the prescription compatibility principle of traditional Chinese medicine (TCM) formulations and determining the dosage and usage of each medicine [34]. The components of Chinese herbal formulas are complex and diverse, and the main treatment mechanism seems reasonable. These herbal formulas are an organic combination of many effective components, which have a multi-target effect on the disease in the body by multiple pathways [35]. An increasing number of Chinese herbal formulas have been reported to have significant anti-AP effects, and have become a treatment option in many hospitals for AP [36–38].
Pharmacologic studies have demonstrated that Tetramethylpyrazine (TMP, also known as Ligustrazine), an intravenous drug made from traditional Chinese herbs, is able to reduce inflammatory responses [39] and activation of apoptosis [40]. It is capable of attenuating the severity of AP and protecting multiple organ injury in rats with AP [41]. Our results demonstrate that cerulein-induced AP shows a more acute pancreatitis model with deteriorated pancreatic inflammation, increased serum cytokine level, evident local acinar necrosis, as well as drastic systemic inflammatory responses. Importantly, all parameters quantifying the severity of AP were reduced, but cell apoptosis was increased in TMP-treated AP as compared with the cerulein-induced AP.
Many factors are involved in the process of the pathogenesis of AP, and the accurate mechanisms are still unclear. Evidences suggest that proinflammatory cytokines, such as IL-1β, TNF-α, and IL-6, act as mediators of acute pancreatitis. IL-6 is a proinflammatory cytokine associated with acute phase responses during inflammation, and elevated levels of IL-6 have been observed in patients with AP and are determinants of disease severity [42]. TNF-α is produced in pancreatic acinar cells in experimental AP model. It is an activator of immune cells and regulates the synthesis of other pro-inflammatory cytokines [43]. It is critical for the therapy of AP by reducing the cascade of cytokines at the early stages and ameliorating the disease and its systemic complications. The cerulein-induced pancreatitis was a sudden inflammation in the pancreas with extensive infiltration of leukocytes and excessive production of amylase. Dang et al. have reported that TMP can ameliorate microcirculatory disorder and alleviate the damage to the pancreas and stomach [44]. Zhang et al. have reported that TMP can ameliorate the condition of microcirculatory disorder and the damage of pancreas and kidney [45]. In this study, TMP greatly reduced the infiltration of leukocytes and pancreas damage and decreased the IL-6 and TNF-α level in AP models by inhibiting the activation of NF-κB. Inhibition of NF-κB has been shown to improve survival rates in rats with taurocholate-induced pancreatitis [46]. Therefore, treatment with TMP could be an efficacious and promising remedy in the treatment for AP.
Apoptosis in pancreatic acinar cells is mediated mainly by activation of caspases. Of importance, it has been increasingly recognized that caspases not only mediate apoptosis but also protect from necrosis and decrease the severity of pancreatitis [45]. NF-κB-dependent anti-apoptotic gene transcriptional activation and caspase system inhibition has been demonstrated to be crucial in regulating cell death in pancreatitis [6,47]. Chen et al. have reported that TMP alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways [48]. Our findings indicated that TMP reduced the activation of NF-kB and increased the subsequent cleaved-caspase-3 expression and reduced the subsequent bcl-2 expression, which resulted in increased apoptosis and reduced severity of cerulein-induced AP.
In conclusion, our study demonstrates that TMP could protect against AP by reducing the activation of NF-κB,blocking inflammatory factors and increasing cell apoptosis, thereby collectively reduced the severity of AP. Thus, TMP represents a potential therapeutic agent in the management of AP.
Funding Statement
This work was founded by Shandong Natural Scientific Research Fund [funding reference no. ZR2015HM1007].
Highlights
Tetramethylpyrazine (TMP) effectively reduced the cerulein-induced pancreatic histological damage.
Tetramethylpyrazine inhibits amylase activity and pro-inflammatory cytokines TNF-α and IL-6.
Tetramethylpyrazine blocks NF-κB activation and inhibits of pro-apoptotic signals in AP models.
Authors’ contributions
CLY and ZJL designed the project and performed all the experiments. CYJ and HY wrote the article. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethics approval and consent to participate
No human subjects were used in this research.
References
- [1].Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. [DOI] [PubMed] [Google Scholar]
- [2].Tenner S, Baillie J, DeWitt J, et al. American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. 2013;108:1400–15;1416. [DOI] [PubMed] [Google Scholar]
- [3].Steinle AU, Weidenbach H, Wagner M, et al. NF-kappaB/Rel activation in cerulein pancreatitis. Gastroenterology. 1999;116:420–430. [DOI] [PubMed] [Google Scholar]
- [4].Bhatia M. Novel therapeutic targets for acute pancreatitis and associated multiple organ dysfunction syndrome. Curr Drug Targets Inflamm Allergy. 2002;1:343–351. [DOI] [PubMed] [Google Scholar]
- [5].Gukovskaya AS, Pandol SJ. Cell death pathways in pancreatitis and pancreatic cancer. Pancreatology. 2004;4:567–586. [DOI] [PubMed] [Google Scholar]
- [6].Mareninova OA, Sung KF, Hong P, et al. Cell death in pancreatitis: caspases protect from necrotizing pancreatitis. J Biol Chem. 2016;281:3370–3381. [DOI] [PubMed] [Google Scholar]
- [7].Bhatia M. Apoptosis vs. necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:G189–G196. [DOI] [PubMed] [Google Scholar]
- [8].Hahm KB, Kim JH, You BM, et al. Induction of apoptosis with an extract of Artemisia asiatica attenuates the severity of cerulein-induced pancreatitis in rats. Pancreas. 1998;17:153–157. [DOI] [PubMed] [Google Scholar]
- [9].Kaiser AM, Saluja AK, Sengupta A, et al. Relationship between severity, necrosis, and apoptosis in five models of experimental acute pancreatitis. Am J Physiol. 1995;269:C1295–C1304. [DOI] [PubMed] [Google Scholar]
- [10].Bhatia M, Wallig MA, Hofbauer B, et al. Induction of apoptosis in pancreatic acinar cells reduces the severity of acute pancreatitis. Biochem Biophys Res Commun. 1998;246:476–483. [DOI] [PubMed] [Google Scholar]
- [11].Beg AA, Sha WC, Bronson RT, et al. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. [DOI] [PubMed] [Google Scholar]
- [12].Burstein E, Duckett CS. Dying for NF-kB? Control of cell death by transcriptional regulation of the apoptotic machinery. Curr Opin Cell Biol. 2003;15:732–737. [DOI] [PubMed] [Google Scholar]
- [13].Dutta J, Fan Y, Gupta N, et al. Current insights into the regulation of programmed cell death by NF-kappaB. Oncogene. 2006;25:6800–6816. [DOI] [PubMed] [Google Scholar]
- [14].Liu HT, Du YG, He JL, et al. Tetramethylpyrazine inhibits production of nitric oxide and inducible nitric oxide synthase in lipopolysaccharide-induced N9 microglial cells through blockade of MAPK and PI3K/Akt signaling pathways, and suppression of intracellular reactive oxygen species. J Ethnopharmacol. 2010;129:335–343. [DOI] [PubMed] [Google Scholar]
- [15].Li J, Zhang S, Zhou R, et al. Perspectives of traditional Chinese medicine in pancreas protection for acute pancreatitis. World J Gastroenterol. 2017;23:3615–3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Xiang H, Zhang Q, Qi B, et al. Chinese herbal medicines attenuate acute pancreatitis: pharmacological activities and mechanisms. Front Pharmacol. 2017;8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen L, Liu T, Wang Q, et al. Anti-inflammatory effect of combined tetramethylpyrazine, resveratrol and curcumin in vivo. BMC Complement Altern Med. 2017;17:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gong X, Ivanov VN, Hei TK. 2,3,5,6-Tetramethylpyrazine (TMP) down-regulated arsenic-induced heme oxygenase-1 and ARS2 expression by inhibiting Nrf2, NF-κB, AP-1 and MAPK pathways in human proximal tubular cells. Arch Toxicol. 2016;90:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhu X, Wang K, Zhang K, et al. Tetramethylpyrazine protects retinal capillary endothelial cells (TR-iBRB2) against IL-1β-induced nitrative/oxidative stress. Int J Mol Sci. 2015;16:21775–21790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, Fu Q, Zhao W. Tetramethylpyrazine inhibits osteosarcoma cell proliferation via downregulation of NF-κB in vitro and in vivo. Mol Med Rep. 2013;8:984–988. [DOI] [PubMed] [Google Scholar]
- [21].Nathan JD, Romac J, Peng RY, et al. Transgenic expression of pancreatic secretory trypsin inhibitor-I ameliorates secretagogue-induced pancreatitis in mice. Gastroenterology. 2005;128:717–727. [DOI] [PubMed] [Google Scholar]
- [22].Wildi S, Kleeff J, Mayerle J, et al. Suppression of transforming growth factor beta signalling aborts caerulein induced pancreatitis and eliminates restricted stimulation at high caerulein concentrations. Gut. 2007;56:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu W, Yu X, Luo XP, et al. Tetramethylpyrazine protects against scopolamine-induced memory impairments in rats by reversing the cAMP/PKA/CREB pathway. Behav Brain Res. 2013;253:212–216. [DOI] [PubMed] [Google Scholar]
- [24].Zhang JX, Dang SC, Qu JG, et al. Preventive effect of tetramethylpyrazine on intestinal mucosal injury in rats with acute necrotizing pancreatitis. World J Gastroenterol. 2006;12:6386–6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gukovsky I, Gukovskaya AS, Blinman TA, et al. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:G1402–G1414. [DOI] [PubMed] [Google Scholar]
- [26].Ji B, Tsou L, Wang H, et al. Ras activity levels control the development of pancreatic diseases. Gastroenterology. 2009;137:1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wu DM, Wang S, Shen M, et al. S100A9 gene silencing inhibits the release of pro-inflammatory cytokines byblocking the IL-17 signalling pathway in mice with acute pancreatitis. J Cell Mol Med. 2018;22:2378–2389. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [28].Weerasinghe SV, Moons DS, Altshuler PJ, et al. Fibrinogen-γ proteolysis and solubility dynamics during apoptotic mouse liver injury: heparin prevents and treats liver damage. Hepatology. 2011;53:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kubisch C, Dimagno MJ, Tietz AB, et al. Overexpression of heat shock protein Hsp27 protects against cerulein-induced pancreatitis. Gastroenterology. 2004;127:275–286. [DOI] [PubMed] [Google Scholar]
- [30].G C N, Arnaoutis TP, Giamarellos-Bourboulis EJ. The significance of the dosage adjustment of octreotide in the treatment of acute pancreatitis of moderate severity. Hepatogastroenterology. 2001;48:1754–1757. [PubMed] [Google Scholar]
- [31].Krishnan K. Nutritional management of acute pancreatitis. Curr Opin Gastroenterol. 2017;33:102106. [DOI] [PubMed] [Google Scholar]
- [32].Dimagno MJ. Clinical update on fluid therapy and nutritional support in acute pancreatitis. Pancreatology. 2015;15:583588. [DOI] [PubMed] [Google Scholar]
- [33].Zhang X, Zhang J, Yang P. Research status of the mechanism and treatment for acute pancreatitis complicated with hepatic injury. J Nanjing Med Univ. 2008;22:199204. [Google Scholar]
- [34].Yao H, Qiao YJ, Zhao YL, et al. Herbal medicines and nonalcoholic fatty liver disease. World J Gastroenterol. 2016;22:68906905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Xiang H, Wang G, Qu J, et al. Yin-Chen-HaoTang attenuates severe acute pancreatitis in rat: an experimental verification of in silico network target prediction. Front Pharmacol. 2016;7:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Zhong K. Curcumin mediates a protective effect via TLR-4/NF-kappaB signaling pathway in rat model of severe acute pancreatitis. Cell Biochem Biophys. 2015;73:175–180. [DOI] [PubMed] [Google Scholar]
- [37].Zhang Y, Zhu L, Wang J, et al. Formula compatibility identification of Dachengqi decoction based on the effects of absorbed components in cerulein-injured pancreatic AR42J cells. Evid Based Complement Alternat Med. 2016;2016:3198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang X, Tian H, Wu C, et al. Effect of baicalin on inflammatory mediator levels and microcirculation disturbance in rats with severe acute pancreatitis. Pancreas. 2009;38:732–738. [DOI] [PubMed] [Google Scholar]
- [39].Wang Z, Zhou Z, Wei X, et al. Therapeutic potential of novel twin compounds containingTetramethylpyrazine and carnitine substructures in experimental ischemic stroke. Oxid Med Cell Longev. 2017;2017:7191856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Pan J, Shang JF, Jiang GQ, et al. Ligustrazine induces apoptosis of breast cancer cells in vitro and in vivo. J Cancer Res Ther. 2015;11:454–458. [DOI] [PubMed] [Google Scholar]
- [41].Zhang XP, Wang C, Wu DJ, et al. Protective effects of ligustrazine, kakonein and Panax notoginsenosides on multiple organs in rats with severe acute pancreatitis. Methods Find Exp Clin Pharmacol. 2010;32:631–644. [DOI] [PubMed] [Google Scholar]
- [42].Gregoric P, Sijacki A, Stankovic S. SIRS score on admission and initial concentration of IL-6 as severe acute pancreatitis outcome predictors. Hepato-Gastroenterology. 2010;57:349–353. [PubMed] [Google Scholar]
- [43].Wang XY, Tang QQ, Zhang JL, et al. Effect of SB203580 on pathologic change of pancreatic tissue and expression of TNF-alpha and IL-1beta in rats with severe acute pancreatitis. Eur Rev Med Pharmacol Sci. 2014;10:338–343. [PubMed] [Google Scholar]
- [44].Dang SC, Zhang JX, Qu JG, et al. Ligustrazine alleviates gastric mucosal injury in a rat model of acute necrotizing pancreatitis. Hepatobiliary Pancreat Dis Int. 2007;6:213–218. [PubMed] [Google Scholar]
- [45].Zhang JX, Dang SC, Qu JG, et al. Ligustrazine alleviates acute renal injury in a rat model of acute necrotizing pancreatitis.World. J Gastroenterol. 2006;12:7705–7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Satoh A, Shimosegawa T, Fujita M. Inhibition of nuclear factor-κB activation improves the survival of rats with taurocholate pancreatitis. Gut. 1999;44:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pandol SJ, Saluja AK, Imrie CW, et al. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132:1127–1151. [DOI] [PubMed] [Google Scholar]
- [48].Chen J, Chen J, Wang X, et al. Ligustrazine alleviates acute pancreatitis by accelerating acinar cell apoptosis at early phase via the suppression of p38 and Erk MAPK pathways. Biomed Pharmacother. 2016;82:1–7. [DOI] [PubMed] [Google Scholar]


