Abstract
Although it is intuitive that antibiotics administered before obtaining a blood culture would reduce the likelihood of obtaining a positive culture, it is not clear exactly how rapidly and to what extent blood becomes sterile after administration of intravenous (IV) antibiotics. Using a large data set of patients admitted from the UFHealth Shands Adult Emergency Department (ED) between 2012 and 2016 (n = 25 686), we had the opportunity to more closely examine the effect of starting IV antibiotics before vs after obtaining blood cultures. We present data on the effect of pretreatment with IV antibiotics for both septic and nonseptic ED patients on the blood culture positivity rate on an hour-by-hour basis, as well as the effects on distribution of species recovered and the impact of antibiotic resistance in empiric treatment with antibiotics.
Keywords: antibiotic pretreatment, blood cultures, blood culture positivity, sepsis
Obtaining blood cultures after starting intravenous (IV) antibiotics is inherently likely to reduce the yield of positive cultures, and obtaining blood cultures before starting antibiotics is one of the cornerstones of the Surviving Sepsis Campaign (SSC) Hour-1 bundle [1]. Data from the SSC show that obtaining blood cultures before starting antibiotics is associated with improved survival [1, 2]. Pruinelli et al. [3], modeling the SSC elements, found that there was no loss of improved outcome if blood cultures were delayed up to 50 minutes after starting IV antibiotics, but the improvement in mortality of SSC does not bear on the question of culture positivity.
Previous studies have found significant reductions in blood culture positivity rates during antibiotic treatment, but they have studied wide time periods of 1–3 days after antibiotics were started [4, 5]. These studies showed reductions in positivity rates of 54%–55% overall, with higher recovery of Staphylococcus aureus compared with gram-negative bacteria in the cultures obtained while receiving antibiotics. Our study is unique in that we were able to look at the initial 6-hour time frame after administration of IV antibiotics on an hourly basis and document that most of this reduction in positivity occurs as early as 1–2 hours after antibiotic treatment is started.
METHODS
Study Population
All adult patients (≥18) with blood cultures obtained in the emergency department (ED) who were admitted from the UFHealth Shands ED between August 1, 2012, and December 31, 2016, were included in the study (n = 25 686). Hospital–hospital transfers and patients who never received IV antibiotics were excluded. The patients were 50% male, 70% white, 25.1% black, and 4.9% other or unknown. The mean ± SD age was 57.1 ± 18 years. There were 6731 septic patients with a mean age of 59.2 ± 17.5 years and 18 955 nonseptic patients with a mean age of 56.4 ± 18.1 years. Sepsis was defined based on coding after discharge as present on admission (POA). This study was approved by the University of Florida Institutional Review Board (IRB201701456).
Study Methods
The following data were obtained from the electronic medical record:
Arrival date and time to the ED, time to first IV antibiotic dose after arrival in the ED, time to first blood culture, organism from blood culture results if positive, antibiotic susceptibility, antibiotic(s) used to treat the patient, and whether the patient had sepsis present on admission, based on UHC (now Vizient) discharge coding.
Blood Culture Methods
Blood cultures were incubated in BacTec (Becton, Dickinson, Inc, Franklin Lakes, NJ) aerobic, anaerobic, and pediatric resin bottles for up to 5 days or until they signaled as positive. We considered S. aureus, gram-negative rods, and beta-hemolytic Streptococci and enterococci as significant pathogens, and we excluded coagulase-negative staphylococci, S. viridans, Propionibacterium sp, Micrococcus sp, and Bacillus sp as contaminants. The choice of this group of organisms was based on the review of blood culture contamination by Hall and Lyman [6]. Susceptibility testing was performed phenotypically on the Vitek II (bioMerieux, Durham, NC). Active antibiotics were defined as an antibiotic to which the organism did not display phenotypic resistance.
Data Analysis
We calculated the hourly rate of positive blood cultures obtained before and after the start of IV antibiotics by subtracting the time stamp in the electronic medical record (Epic, Madison, WI) between the first blood culture collection time and the start of the first IV antibiotic dose. For example, the first hour before IV antibiotics were started was considered 60 minutes–1 minute before antibiotics were started, and after antibiotics were started, the hour was 0–59 minutes after the start of IV antibiotics. Blood culture collection times were hand written on the blood culture bottles by the nurses in the ED at collection, and the times were entered into Epic when accessioned by the laboratory. Chi-square and t tests were used to calculate statistical significance for the percentage of positive blood cultures before vs after the start of IV antibiotics, and for mortality percentage before and after IV antibiotics.
RESULTS
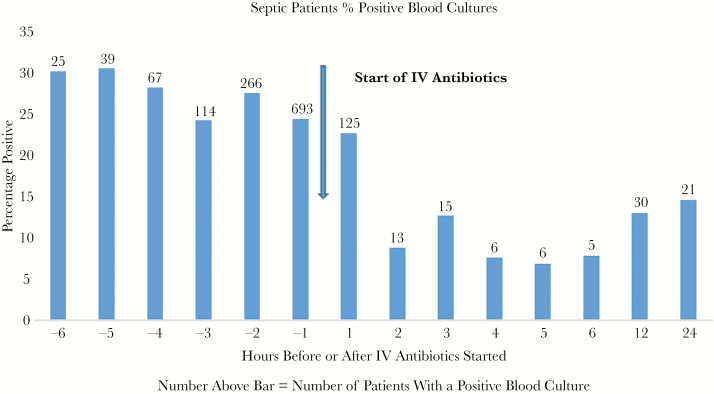
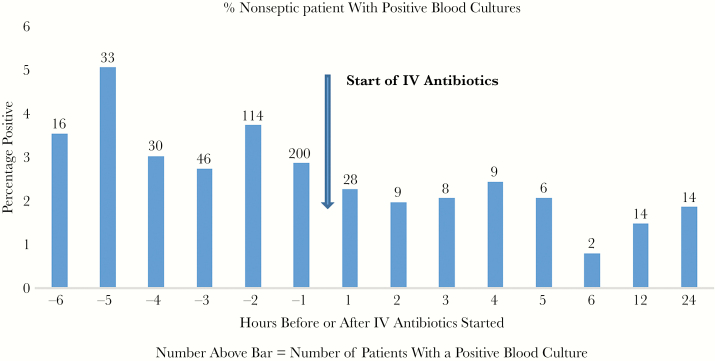
The percentage of septic patients with a positive blood culture within 1 hour after starting IV antibiotics was 22.6% vs 24.3% for the 1 hour before (P = .1064, NS). Thereafter, the percent positive fell dramatically to an average of 9.1% over the next 5 hours (Figure 1). Nonseptic patients averaged 3.5% positive for the 6 hours before IV antibiotics vs 1.9% for the 6 hours after (P = .008) (Figure 2).
Figure 1.
The bar graph shows the percentage of septic patients with positive blood cultures for each hour before starting antibiotics, for each hour after (up to 6 hours), and for 6–12 and 12–24 hours. The actual number of patients with positive blood cultures is shown above the bar. The arrow indicates the start of intravenous (IV) antibiotics.
Figure 2.
The bar graph shows the percentage of nonseptic patients with positive blood cultures for each hour before starting antibiotics, for each hour after (up to 6 hours), and for 6–12 and 12–24 hours. The actual number of patients with positive blood cultures is shown above the bar. The arrow indicates the start of intravenous (IV) antibiotics.
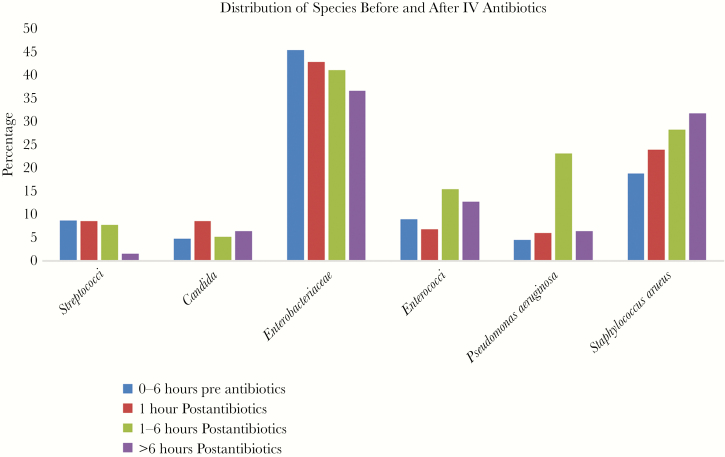
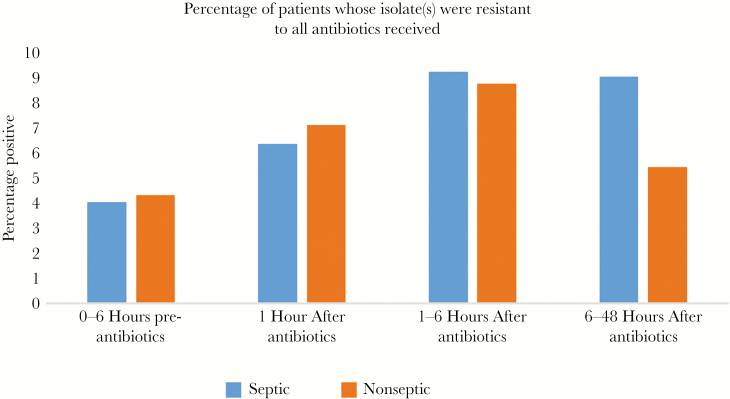
As shown in Figure 3, there is a shift in the distribution of species recovered before vs after starting IV antibiotics, with a decreasing percentage of Enterobacteriaceae and an increasing percentage of Staphylococcus aureus, P = .2157 (NS) that was not statistically significantly different for Staphylococcus aureus vs Enterobacteriaceae, but was statistically significantly different P = .007126 for the entire distribution, chi-square. Figure 4 shows the percentage of patients whose isolates were resistant to all IV antibiotics they received before vs after IV antibiotics were begun. After starting IV antibiotics there is an increase in the percentage of patients receiving no active antibiotics, but it never exceeded 10% of all isolates and did not reach statistical significance (septic P = .09562 and nonseptic P = .616, chi-square).
Figure 3.
The bar graph shows the percentage distribution of positive blood cultures by species of bacteria and yeast for 0–6 hours before starting antibiotics (blue), for the first hour (red) after starting intravenous (IV) antibiotics, and for 1–6 hours (green) and >6 hours (purple) after starting IV antibiotics.
Figure 4.
The bar graph shows the percentage of patients whose isolates were resistant to all treatment antibiotics for septic (blue) and nonseptic (red) patients for each time period. Abbreviation: IV, intravenous.
DISCUSSION
As would be expected, there is a significant decrease in the percentage of blood cultures positive for clinically likely pathogens starting 1 hour after IV antibiotics were begun both for septic and non-septic patients. As our patients did not have serial blood cultures done, we cannot know exactly what the rate of positive blood cultures would have been before IV antibiotics were started. Nonetheless, even with different patient groups in each hourly time frame, the data are consistent with previously published rates of blood culture positivity for septic patients before and during antibiotic treatment [4, 5]. Our study differed in several respects from those of Grace et al. [5] and of Scheer et al. [4]. The patients studied by Grace et al more closely resembled our ED patient population, in that they specifically studied patients admitted for “suspected infection” and they excluded patients in the intensive care units. Their patients were included if cultures were obtained within 24 hours before and up to 72 hours after starting antibiotics, but their positivity rates were not broken down into narrower time intervals. Scheer’s patient population was septic intensive care patients, as opposed to adult ED patients in our study. They included patients up to 36 hours before starting antibiotic treatment. In our ED population, we considered blood cultures obtained >6 hours before starting antibiotics in the ED as noninterpretable in the context of an acute admission to the ED. Their antibiotic treatment group included patients up to 36 hours after starting antibiotics, but positivity rates were not reported for time frames with that period. Our data on the hourly positivity rates therefore complement both studies. Grace et al did not report mortality data for their patients, but Scheer et al noted that despite a lower rate of blood culture positivity, the corresponding mortality was not statistically different between those with blood cultures obtained before vs after starting antibiotics. We found similar results, in that hourly mortality was closely correlated with UHC (Vizient) expected mortality and was essentially unaffected in patients whose cultures were obtained after antibiotics were started (data not shown) [7, 8].
Antibiotic treatment also appears to shift the distribution of species recovered (Figure 3) most obviously from Enterobacteriaceae to Staphylococcus aureus on a percentage basis. This shift was also observed in the study of Grace et al [3] who found that Staphylococcus aureus declined 24% in antibiotic treated patients, whereas gram negative organisms declined 88%. It may be that the patient population studied by Grace et al as noted above was more like ours than that of Scheer et al. Both Grace et al. [5] and Scheer et al. [4] reported overall decreases of 54%–55% in positivity rate when patients were receiving antibiotics, but the percentage decrease was about the same for both Staphylococcus aureus and gram negatives in Scheer’s study.
For patients receiving antibiotics, there was an increased percentage of isolates resistant to all treatment antibiotics. However, resistant isolates never accounted for more than 10% of the total isolates recovered, and thus do not explain the ongoing positivity rate after the start of IV antibiotics.
Some limitations of our study include being a single-center study that could limit generalization. However, in view of the studies discussed above, our experience is consistent with those in other academic settings. Another limitation is that we did not have paired blood cultures before and after starting antibiotics from the same group of patients. Scheer et al do report that a small group of 35 of their patients did have blood cultures both before and after antibiotics were started and had 20/35 (57.1%) positive before starting antibiotics and 9/35 (25.7%) after, consistent with the overall decrease in their nonpaired patients as well as the study by Grace et al. and our report here.
CONCLUSIONS
The rate of positive blood cultures is reduced by >50% for both septic and nonseptic patients as early as the second hour of IV antibiotic treatment, consistent with earlier studies of blood cultures obtained up to 36–72 hours after starting antibiotic treatment. Antibiotic resistance did not explain the ongoing positivity rate, and the distribution of species decreased percentage-wise for Enterobacteriaceae and increased for Staphylococcus aureus. For patients who do not have blood cultures obtained before starting antibiotics, it is still worthwhile to obtain them, especially in the setting of sepsis.
Acknowledgments
We gratefully acknowledge the support of the Department of Pathology, Immunology and Laboratory Medicine, Department of Emergency Medicine, and Office of Chief Information Officer, UFHealth Shands Hospital, University of Florida, Gainesville, Florida.
Financial support. This work was supported in part by internal funds from the Department of Pathology, Immunology and Laboratory Medicine, Department of Emergency Medicine, and Office of Chief Information Officer, UFHealth Shands Hospital, University of Florida, Gainesville, Florida.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Prior presentation. Presented in part at ASMicrobe; June 7–11, 2018; Atlanta GA; and at IDWeek; October 3–7, 2018; San Francisco, CA.
References
- 1. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Crit Care Med 2018; 46:997–1000. [DOI] [PubMed] [Google Scholar]
- 2. Levy MM, Rhodes A, Phillips GS, et al. . Surviving sepsis campaign: association between performance metrics and outcomes in a 7.5-year study. Crit Care Med 2015; 43:3–12. [DOI] [PubMed] [Google Scholar]
- 3. Pruinelli L, Westra BL, Yadav P, et al. . Delay within the 3-hour surviving sepsis campaign guideline on mortality for patients with severe sepsis and septic shock. Crit Care Med 2018; 46:500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scheer CS, Fuchs C, Gründling M, et al. . Impact of antibiotic administration on blood culture positivity at the beginning of sepsis: a prospective clinical cohort study. Clin Microbiol Infect 2019; 25:326–31. [DOI] [PubMed] [Google Scholar]
- 5. Grace CJ, Lieberman J, Pierce K, Littenberg B. Usefulness of blood culture for hospitalized patients who are receiving antibiotic therapy. Clin Infect Dis 2001; 32:1651–5. [DOI] [PubMed] [Google Scholar]
- 6. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev 2006; 19:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rand KH, Beal SG, Rivera K, et al. . Should the blood culture requirement in the sepsis core measure be revised? Paper presented at: ASMicrobe; June 7–11, 2018; Atlanta GA. [Google Scholar]
- 8. Rand KH, Beal SG, Rivera K, et al. . If blood cultures were not done before starting antibiotics, is it of any value to obtain them later? Paper presented at: IDWeek; October 3–7, 2018San Francisco, CA. [Google Scholar]