Abstract
Background and objectives:
The Francophone Africa Transfusion Research Network conducted the first large and comprehensive surveys on the status of blood safety in francophone African countries in 2009 and 2012. Since then there has been substantial investment in blood safety but little is known about progress made in the region.
Materials and Methods:
This multicenter cross sectional study describes characteristics of blood services, donors and blood products and compare results with previous data. A web-based questionnaire collected data of 2016 from 38 blood facilities in 11 French-speaking countries. Data were analyzed according to type of blood services and compared to similar studies conducted in 2009 and 2012.
Results:
The study included data on 572,933 donations from 366,844 donors. Compared to 2012, there was an increase in the median proportion of voluntary non remunerated blood donation (+22%) (p=0.004), and a reduction from 2.1 to 0.9 (p=0.01), from 10.3 to 6.7 (p=0.00), from 3.2 to 1.3 (p=0.006) and from 1 to 0.4 (p=0.03) in median seroprevalences of HIV, HBV, HCV and syphilis, respectively. The median proportion of blood orders fulfilled decreased (−18.2%) (p=0.001). The number of technical staff per 1,000 donations ranged from 1 to 54 with hospital-based blood transfusion services having 12-fold more staff than National and Regional services.
Conclusion:
Several indicators have improved in Francophone Africa Blood services during the last 5 years and national and regional services likely have better indicators than hospital-based services. These findings may support the need for ongoing blood safety initiatives.
Keywords: blood donor, donated blood, Africa, survey
Introduction
Despite notable improvement in the last decades, the lack of blood to treat severe perinatal hemorrhage contributes to up to 72% of maternal deaths [1] and the mean HIV residual risk is 1/29,000 blood donations in several African countries [2]. Significant gaps remain in safe blood availability and in employing appropriate clinical transfusion guidelines. As different regions have different levels of development and infrastructure, with significant interregional variability also, each has specific challenges to the availability and safety of blood [3].
In 2008 and 2011, French-speaking countries had a high overall positive rates of transfusion-transmitted infections (TTI) of 12.8% and a low proportion of blood orders fulfilled of 50% as reported by the Francophone Africa Transfusion Research Network [4,5]. There was a limited level of implementation of quality systems in most countries especially in services using rapid tests for TTI screening [6,7].
To address these deficiencies, the network supported participating blood services through advisory meetings, external quality assessments, regular individual staff training and mentorship [8]. Some technical assistance such as training, capacity building and quality systems implementation were also provided by Ministries of public health with technical support from Safe blood for Africa Foundation, WHO, CDC/PEPFAR, Global fund, Safe Blood for Africa Foundation or Africa Society for Blood Transfusion (AfSBT) [9,10].
Since that time, a systematic reassessment has not been conducted in Francophone Africa blood services to measure progress made. In addition, safety indicators have not been compared between hospital-based blood banks and centralized blood transfusion services.
We conducted a study to provide contemporary data on blood services, donors and blood products within the Francophone research network and compare them with previous data collected in the same region.
Methods
Study design
We conducted a multicenter cross-sectional survey on characteristics of blood transfusion services which were members of the Francophone Africa Transfusion network during calendar year 2016 and compared the new data to previously published results from similar surveys in 2009 and 2012 [4,5].
Subjects and sampling
An invitation to participate was emailed to all the 51 network member blood services. The 51 members included nine National Blood Service (NBS), 17 Regional Blood Service (RBS), 20 Hospital-based blood bank (HBB) and 5 collection sites (CS). We enrolled 38 blood facilities amongst the 51 in the network based only to their willingness to participate. The blood services enrolled were either a National service, regional service, hospital-based blood bank or a collection site. A collection site is a small blood service generally located in small cities or remote areas that only collects and store blood while testing is additionally performed in hospital-based, regional and national services. A national blood service cover blood safety activities at national level while regional services cover a region under national service authority. Hospital-based service are under authority of hospital in which they are located while regional and national services are autonomous.
Questionnaire and data collection
Data were collected for the period 1st January 2016 to 31st December 2016. A web-based questionnaire was developed and sent to all members of the research network to collect data. It was self-administered and structured to obtain standard blood safety data in 4 specific domains according to type of blood services:
Administrative characteristics relevant to blood safety: these includes type of staff, different services provided by the institution (donor program, blood collection and coverage of blood order, infectious disease screening including the type and proportion of units tested for each TTI, the proportion of units of blood tested for blood grouping; preparation and distribution of blood products, preparation of components; use of guidelines; use of electronic information systems); existence of a quality management system for transfusion activities; and technical/financial assistance by a foreign or international organization.
The characteristics of the blood donors and management of the blood supply including the proportion of mobile collection sites; the numbers of blood donors per year; the frequency of donation— distribution of sex, age, new versus regular (two or more donations) donor, type of donor (voluntary, familiar/replacement, remunerated, autologous), and type of donation (regular, first-time);
The characteristics of the blood donation including the frequency of RBC phenotypes in the ABO and Rh systems; testing for and prevalence of transfusion-transmitted infections (TTI): HIV, hepatitis B virus [HBV], hepatitis C virus [HCV], plasmodium species, Treponema palladum and other infectious agents).
The testing algorithms used in TTI screening and blood group serology and; positive rates of transfusion-transmissible infections based on screening (not confirmatory) test results;
Participants collected data retrospectively from their records and filled out the online questionnaire over a maximum of 3 months. The head of each blood center first reviewed and approved data prior to submission. Centers were asked to complete incomplete data in follow-up emails.
Data analysis
Data were entered in Excel 6.0. Data were analyzed according to the type of blood service: national blood service, regional blood service, hospital-based blood service and collection site. The following outcomes was calculated: proportion of blood orders fulfilled, proportion of mobile collection, proportion of voluntary non-remunerated blood donation (VNRBD), proportion of deferred donors, TTI positive rates, proportion of whole blood issued. Means, median and ranges of key blood safety characteristics were calculated for each data where applicable and distributed according to the four main types of blood center. We compared the medians using Chi-squared tests. A difference in the results with a P-value <0.05 was considered significant.
The study was exempt from institutional review board approval because only summary data without identifying information on donors was collected. Nevertheless each participating blood service reviewed the protocol and the questionnaire and provided a written agreement to participate. Blood service names are not cited in this report.
Results
Study population
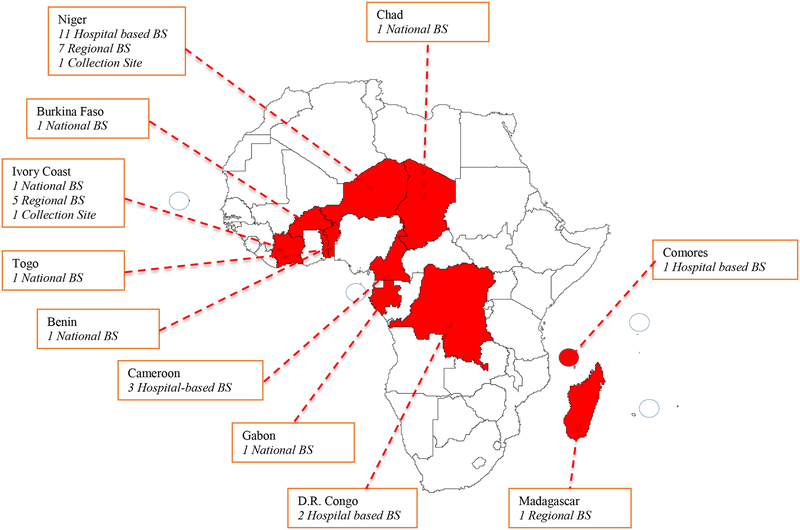
A total of 38 blood services from 11 francophone countries participated in the survey including six National blood services, 13 Regional services, 17 hospital-based blood services, and two collection sites. Blood transfusion services from eleven sub-Saharan African countries participated in the study: four from Central Africa (Cameroon, Congo-DRC, Gabon, Chad), five from Western Africa (Benin, Burkina Faso, Ivory Coast, Niger, Togo), and two from Southern Africa (Comores, Madagascar) (Fig 1).
Figure 1:
Number, type and country location of the 38 participating blood services (BS)
These services collected 572,933 units from 366,844 blood donors during the study period. Fulfillment of blood orders ranged from 20.5 to 100% with the highest proportion in national blood services (75.5%) (Table 1). The mean proportion of VNRBD was higher in collection sites and lower in hospital-based blood services. The positives rates of HIV, HBV, HCV and Syphilis reported the participating services were 1.4(0.2–3.7), 6.2 (0.4–11.1), 1.6(0.1–5.8), 1 (0.0–5.1) respectively.
Table 1:
Characteristics of donors and donations in the 38 Francophone Africa blood services according to type of center in 2016.
| Characteristics | National Blood Services (n=6) | Regional Blood Services (n=13) | Hospital-based blood services (n=17) | Collection sites (n=2) | All services (n=38) | |
|---|---|---|---|---|---|---|
| Number of blood donors | 266,627 | 70,015 | 26,071 | 4,131 | 366,844 | |
| Annual number of donations | 425,425 | 104,395 | 38,362 | 4,751 | 572,933 | |
| Proportion of Blood order fulfilment% (median (range)) | 75.5 (71–100) | 75.8 (20.5–100) | 51.8 (23.3–100) | na | 63.8 (20.5–100) | |
| Proportion of Mobile collection% (median (range)) | 48(6.6–59.4) | 55(0–61.8) | 84 (0–95.9) | 42.1 | 80.6 (0–95.9) | |
| Proportion of Voluntary Non Remunerated donors% (median (range)) | 78 (14–100) | 81.4 (32–100) | 55 (5–95) | 100 | 83(5–100) | |
| Proportion of Regular donors% (median (range)) | 68.2 (53–87) | 41.8 (8–58) | 55.8 (3–95) | 92 | 55.8 (3–95) | |
| Proportion of Deferred donors% (median (range)) | 18.4 (9.2–33.3) | 21.5 (3.1–46.5) | 10.3 (0–66.8) | 14 (1.2–26.8) | 14.7 (0–66.8) | |
| Proportion of Male donors% (median (range)) | 81.3 (73.5–85.7) | 84.4 (75–93) | 78.3 (50.5–91) | 88.9 | 82.1 (50.5–93) | |
| Donors mean Age in Years% (median (range)) | 32(26–42) | 32(21–41) | 30 (22–39) | 20 | 31(21–42) | |
| HIVAb and/or P24Ag positive rate % (median (range)) | 1.6(0.2–2.9) | 1.2(0.2–3.7) | 1.5(0.4–3.9) | 0.6 (0.4–0.7) | 1.4(0.2–3.7) | |
| HBsAg positive rate % (median (range)) | 6.3 (1.7–9.4) | 7.6 (1.1–8.8) | 5.3 (2.2–11.1) | 4(0.4–7.6) | 6.2 (0.4–11.1) | |
| HCV Ab positive rate% (median (range)) | 2(0.7–3.4) | 1.5 (0.3–5.8) | 1.6 (0.1–5.5) | 1.7 (0.1–3.5) | 1.6 (0.1–5.8) | |
| Syphilis Prevalence% (median (range)) | 1.5 (0,3–3.2) | 1.1 (0.0–4.9) | 0.9 (0.5–5.1) | 0.38 (0.0–1.7) | 1 (0.0–5.1) | |
| ABORhD Blood groups frequency% (median (range)) | A | 20.9 (20–28) | 21 (12–24) | 18.3 (12–26.5) | 20 (19–21) | 19.8 (12–26.5) |
| B | 22 (17–28) | 22.1 (12–26) | 16.8 (11–30) | 23 (20–26) | 19.9 (11–30) | |
| AB | 4.2 (1.8–6) | 3.6 (1–5) | 2.8 (1–6) | 4.5 (1–8) | 3.9 (1–8) | |
| O | 49.7 (44–60) | 53.2 (45–75) | 62.2 (50–70) | 52.5 (45–60) | 56.4 (44–75) | |
| RhD | 93.5 (91.4–97.1) | 91.3 (80–95.1) | 94.7 (90–99) | 92.5 (90–95.1) | 93 (80–99) | |
| Proportion of Whole blood separated into components% (median (range)) | 77.0 (15.6–100) | 43(0–100) | 14.4 (0–94) | 50(0–100) | 87 (0–100) | |
Comparison of blood safety characteristics with previous studies
The survey noted a reduction in TTI seroprevalence in the current survey compared to those of 2009 and 2012 (Table 2). Between 2012 and 2017, the median proportion of voluntary non remunerated blood donation, proportion of regular blood donors and mobile collections increased by 22% (p=0.004), 31.8% (p=0.001), and 57.6% (p=0.000) respectively. Between 2009 and 2017, the median seroprevalences of HIV, HBV, HCV and Syphilis decreased from 2.1 to 0.9 (p=0.01), from 10.3 to 6.7 (p=0.00), from 3.2 to 1.3 (p=0.006) and from 1 to 0.4 (p=0.03) respectively. From 2012 and 2017, the median proportion of blood orders fulfilled decreased (−18.2%) (p=0.001) and the proportion of deferred donors increased (+2.7%) (p=0.12).
Table 2:
Median differences between blood safety parameters in 2017 compared to earlier studies in Francophone Africa blood services
| Studies | Median differences between parameters of 2012 and 2017 | p-value | |||
|---|---|---|---|---|---|
| Parameters | Tagny & al, 2009 | Tagny & al, 2012 | Current study, 2017 | na | |
| Date | August 2009 | January 2012 | October 2017 | na | |
| Number of Blood services | 7 | 28 | 38 | na | |
| 62">Number of Countries | 63">7 | 64">15 | 65">11 | 66">na | 67"> |
| Number of Donations | na* | 370,373 | 572,934 | na | |
| Proportion of blood orders fulfilled % (Median (Range)) | na | 82 (27–100) | 63.8 (20.5–178.8) | −18.2 | 0.001 |
| Proportion of Mobile collections % (Median (Range)) | 45 (10–70) | 23 (0–96) | 80.6 (0–95.9) | +57.6 | 0.000 |
| >Proportion of VNRBD % (Median (Range)) | 92 (25.5–100) | 61 (0–100) | 83(5–100) | +22 | 0.004 |
| Proportion of Regular blood donors % (Median (Range)) | 47.3 (24.4–70) | 24 (0–81.6) | 55.8 (3–95) | +31.8 | 0.001 |
| Proportion of Deferred donors % (Median (Range)) | na | 12 (0–36) | 14.7 (0–66.8) | +2.7 | 0.12 |
| Positive rate of HIV % (Median (Range)) | 2.1 (0.8–2.9) | 1.8 (0–7) | 0.9 (0.2–3.7) | −0.9 | 0.01 |
| Positive rate of HBV % (Median (Range)) | 10.3 (2.7–19) | 6.8 (0.7–7.6) | 6.7 (0.4–11.1) | −0.1 | 0.000 |
| Positive rate of HCV % (Median (Range)) | 3.2 (1.4–7) | 2.5 (0–7.5) | 1.3 (0.1–5.8) | −1.2 | 0.006 |
| Positive rate of Syphilis % (Median (Range)) | 1(0.3–9.5) | 1.7 (0–11) | 0.4 (0.0–5.1) | −1.3 | 0.03 |
| Overall positive rate of TTI % (Median(Range)) | 16.6 (5.2–38.4) | 12.8 (0.7–33.1) | 9.3 (0.7–25.7) | −3.5 | 0.001 |
| Proportion of whole blood % (Median (Range)) | 60 (2–100) | na | 87 (0–100) | na |
na: not available or not applicable
Comparison of blood safety characteristics according to type of blood services
Details of comparison of blood services are presented in Table 3. The number of technical staff per 1,000 donations ranged between 1 to 54, with the highest ratio found in hospital-based blood services. Only 2 of 17 hospital-based blood centers had implemented a blood donor recruitment program compared to 6 of 6 for national blood services. The median HIV positive rate was similar in national blood service (1.6%; range 0.2–2.9) and in hospital-based blood services (1.5%; range 0.4–3.9). A total of 21 services (55.2%) prepared blood components among which 5 over 17 Hospital blood banks and 6 over 6 national blood services.
Table 3:
General characteristics of blood safety activities in the 38 Francophone Africa blood services in 2016 according to type of blood service.
| General characteristics | National Blood Services (n=6) | Regional Blood Services (n=13) | Hospital-based blood services (n=17) | Collection sites (n=2) | All services (n=38) |
|---|---|---|---|---|---|
| Number of Senior staff/Center (median (range)) | 25 (4–93) | 1 (0–4) | 2(0–6) | 1 (0–1) | 5 (0–93) |
| †Number of Technical staff /Center (median (range)) | 91 (30–223) | 9(2–26) | 13 (2–50) | 3 (3–4) | 24 (2–223) |
| Number of Technical staff /1,000 donations (median (range)) | 1 (1–2) | 2 (1–5) | 12 (1–54) | 2 (1–3) | 6 (1–54) |
| Number of Blood services doing donor mobilization (n (%)) | 6 | 5 | 3 | 1 | 15 (39.4) |
| Number of services Screening the four main TTI (n (%)) | 6 | 13 | 17 | 2 | 38(100) |
| Number of services doing ABORhD blood grouping (n (%)) | 6 | 13 | 17 | 2 | 38(100) |
| Number of services preparing blood components (n (%)) | 6 | 9 | 5 | 1 | 21(55.2) |
| Number of services having a Hospital Transfusion Committee (n (%)) | 3 | 5 | 2 | 1 | 11 (28.9) |
| Number of services performing EQAS on at least one TTI (n (%)) | 3 | 2 | 6 | 1 | 12 (31.5) |
| Number of services performing EQAS on blood grouping (n (%)) | 3 | 0 | 2 | 1 | 4 (10.5) |
| EQAS collaborators | MoPH,UKNEQAS,SANBS | MoPH,INTS | MoPH,NHLS, CDC, SBFA | MoPH | MoPH,NHLS,CDC, SBFA, INTS, UKNEQAS, SANBS |
| Number of services having Guidelines on Clinical Use of blood (n, (%)) | 3 | 10 | 2 | 1 | 16 (42.1) |
| Number of services referencing on Standards (n (%)) | 3 | 8 | 3 | 1 | 15 (39.4) |
| Types of Standards of reference (types) | ISO 9001/ISO 15189 | ISO 9001/ISO 15189 | ISO 9001/ISO 15189 AfSBT | ISO 9001/ISO 15189 | ISO 9001/ISO 15189 AfSBT |
| Number of services having a Quality Manager (n (%)) | 6 | 6 | 4 | 1 | 17 (44.7) |
| Number of services accredited or certified (n (%)) | 1 | 0 | 0 | 0 | 1 (2.6) |
| Number of services having an Electronic Informatics System (n (%)) | 6 | 5 | 2 | 1 | 14 (36.8) |
AfSBT: Africa Society for Blood Transfusion
CDC: US Services of Disease Control
EQC: External Quality Control
INTS: Institut National de la Transfusion Sanguine, France
MoPH: Local Ministry of Public Health
NHLS: National Health Laboratory Service, South Africa
SBFA: Safe Blood for Africa Foundation, South Africa
SANBS: South African National Blood Service
TTI: Transfusion Transmitted Infection
UKNEQAS: United Kingdom National External Quality Assessment
Technical staff include Senior staff (medical doctors, pharmacists, medical scientists, technologists and nurses)
Secondary findings in donor blood testing techniques
We also found that rapid tests for HIV, HBV, HCV and Syphilis screening were used by 71% to 76.3% of blood services (Table 4) irrespective of the agent. All the 17 hospital –based blood services used rapid testing compared to only 3 of 6 National blood services. No laboratory performed nucleic acid testing.
Table 4:
Number of Francophone Africa blood services using laboratory techniques according to type of blood service in 2016 (N=38).
| Technique | National Blood Services (n=6) | Regional Blood Services (n=13) | Hospital-based blood services (n=17) | Collection sites (n=2) | All services (n=38) | |
|---|---|---|---|---|---|---|
| TTI | n | n | n | n | n(%) | |
| HIV | RT (HIVAb and/or P24Ag) | 2 | 8 | 17 | 1 | 28 (73.6) |
| EIA (HIVAb and/or P24Ag) | 2 | 4 | 3 | 0 | 9 (23.6) | |
| CLIA (HIVAb and/or P24Ag) | 1 | 1 | 1 | 0 | 3 (7.9) | |
| WB | 0 | 2 | 1 | 1 | 4 (10.5) | |
| NAT Testing | 0 | 0 | 0 | 0 | 0 (0) | |
| HBV | RT (HBsAg) | 3 | 8 | 16 | 1 | 28 (73.6) |
| EIA (HBsAg) | 4 | 6 | 4 | 0 | 14 (36.8) | |
| EIA (HBCAb) | 0 | 0 | 1 | 0 | 1 (2.6) | |
| CLIA (HBsAg) | 2 | 2 | 1 | 0 | 5 (13.1) | |
| NAT Testing | 0 | 0 | 0 | 0 | 0 (0) | |
| HCV | RT | 2 | 8 | 16 | 1 | 27 (71.0) |
| EIA | 2 | 5 | 1 | 0 | 9 (23.6) | |
| CLIA | 1 | 2 | 1 | 0 | 4 (10.5) | |
| ImmunoBlot | 0 | 1 | 0 | 1 | 2 (5.2) | |
| NAT Testing | 0 | 0 | 0 | 0 | 0 (0) | |
| Syphilis | RPR | 4 | 9 | 15 | 1 | 29 (76.3) |
| VDRL | 1 | 5 | 1 | 1 | 8 (21.0) | |
| TPHA | 4 | 5 | 4 | 1 | 14 (36.8) | |
| EIA | 0 | 0 | 0 | 1 | 1 (2.6) | |
| Malaria | Thick Smear | 1 | 0 | 3 | 0 | 4 (10.5) |
| RT | 0 | 0 | 1 | 0 | 1 (2.6) | |
| ImmunoFluorescent Assay | 0 | 0 | 1 | 0 | 1 (2.6) | |
| Other TTI | 0 | 0 | 0 | 0 | 0 (0) |
Discussion
Our survey shows an increase of more than 20% in proportion of voluntary donations between 2012 and 2016 and a reduction of more than 7% in overall seroprevalence of transfusion transmitted infections between 2009 and 2016. However hospital-based blood services had worse blood safety indicators compared to regional and national blood services.
When comparing current data with previous observations by our research group [4,5] and WHO report [10], we noted an increase of more than 50% in average proportion of VNRBD and regular blood donation compared to our 2012 survey. Thus the effects of national and international initiatives are apparent in donor recruitment, education, and motivation campaigns. Comparison in donation of 2012 or 2016 data with 2007 data may be less reliable since only 7 facilities were assessed. Similar effects are also seen in reduced TTI prevalences by 1% to 2% compared to 2012. This result can be explained by the increase in the proportion of VNRBD but also by the progressive implementation of good practices during donor deferral in African blood services [11]. Indeed, the challenge of getting safer blood units is not only in recruitment and retention of VNRBD but also in donor deferral as several blood services had not implemented a donor health questionnaire in 2012. High prevalences of TTI were still observed at some services in this survey may be related to a lack of optimal donor deferral based on questionnaires incorporating locally appropriate risk factors [5,12,13]. This study therefore fills the gap in knowledge on blood safety indicators between 2012 and 2017. It is unclear why unmet demand increased despite increase in voluntary donation and retention. This issue could find a possible explanation in the inappropriate patient blood management and clinical use of blood in Africa previously reported [1].
As expected, the hospital-based blood banks do less donor recruitment, produce fewer components and are less likely to have a dedicated quality manager. Hospital-based blood banks have previously been reported to have less donor recruitment, lower proportions of VNRBD and higher TTI prevalence [10,14,15]. The lack of administrative, financial and technical autonomy may lead to lower quality blood safety activities, especially donor management and clinical use of blood, because these activities receive lower priority when blood collection is located in a general laboratory with competing priorities. National and regional services have more numerous senior staff. They are the most trained in blood safety and probably have better experience as reported earlier [16]. African countries that have implemented National Blood services have better blood safety indicators in blood collection, TTI screening and clinical use of blood [10]. However, there is still a large proportion of blood services located inside hospitals and under hospital control. For all these reasons, this study suggests that transfusion services should be preferably set up as autonomous national blood services, especially when blood donor management activities are concerned. However centralized blood banking systems also have considerable financial implications due to the high cost of voluntary donation programs, collection and distribution of blood and products in remote areas. Some evidences showed the benefit of hybrid approach that maintains the progressive establishment of centralized blood banking infrastructure, while simultaneously supporting regional and local hospital transfusion facilities [17].
All participating blood services reported that they screen all their blood products for the four major TTI and conduct ABO Rh blood grouping. These findings are consistent with results from previous studies [5,10] but should not hide huge technical difficulties faced by blood services in TTI screening. Few facilities used EIA technique despite recommendation from our previous studies, probably due to limited funding. Compared to rapid testing, EIA techniques have higher sensitivity in screening TTI markers [6] and the use of rapid testing leads to false negative results and higher residual risk of TTI [2].
To reduce technical errors there is a need to implement at least a minimum quality management systems (QMS), at least those which do not need special financial investment. At this stage of development, very few blood services in Francophone area have adopted the full implementation of a QMS despite support of technical bodies like AfSBT, CDC, AABB or WHO. Few have appointed a quality manager, referred to recognized standards or perform external quality assessment. This is certainly the next most important challenge for African blood services as QMS has been shown to have a large benefit for blood safety [18,19].
Of note, staffing was six times larger in this survey compared to survey of 2011 in which some of the same facilities participated. The number of trained staff per 1.000 donation increased five-fold between 2011 and 2017[16]. This increase could be explained by better training programs and subsequent appointment of trained staff in the facilities under programs conducted by the MoPH and supported by CDC, European Union and WHO. The level of education and experience of staff was not included in the current survey, an omission which should be addressed as these qualities were rather low in the 2011 study [16]. The staffing ratio were calculated by dividing the number of staff working in the blood services by the number of annual donations. The fact that clinical laboratory staff are also working in HBB in a routine and rotation base may explains the higher staffing ratio per 1,000 donation in HBB.
We recognize some limitations in this survey. We did not enroll exactly the same facilities and Francophone countries in the current and 2012 studies. The absence of quality monitored testing and using rapid assays limit the validity of TTIs rates as performance of rapid assays versus confirmatory assays are lower than those of EIAs [6,7]. However, the 2012 study used similar methods and included a similar number and type of participating services in the same group of countries. In addition, participation rates were similar between the 2012 and 2017 surveys, with each including more than 60% of current members of the research group. Thus the measurement of the magnitude of difference between blood safety characteristics recorded in the two studies may reflect a true improvement in francophone blood services. Indeed, in its 2017 survey, WHO also noticed an improvement in Francophone countries between 2010 and 2013 [10]. An additional limitation of this survey is that median differences in key indicators comparing this study to previous ones lacked a confidence interval and data were not broken down by donor age and sex or type of donation. The study did not break down TTI prevalence according to type of donation, age or sex. However, the age and sex distribution of Francophone African donors has not changed much over the last 10 years, namely ages 20 to 30 years with male predominance in most countries [4,12,20].
In summary, this study shows that several measures of blood service quality including TTI prevention and improved blood supply have improved significantly in the last four to five years. It also shows that centralized blood services may have better blood safety indicators compared to hospital-based blood services. Reduced funding of transfusion services over the last years might impact negatively the current progress. We believe these data support the continuation and reinforcement of support to blood safety programs in Francophone Africa. The study also highlight the need of data from local research network for analysis, monitoring and evaluation, and development of appropriate interventions in the field of transfusion safety.
Acknowledgements
CT, SL and EM have designed the study.
CT wrote the manuscript; SL, EM and the Francophone research Network have reviewed the manuscript.
We thank the Heads of African Blood services for the data and the French National Institute for Blood Transfusion (INTS) for supporting the survey.
We also thank the ITAPS mentors (International Traineeship in AIDS Prevention Studies) from the ITAPS Program of University of California, San Francisco) for their great help in reviewing this paper.
Appendix
The Francophone Africa Transfusion Research Network
Sub-Saharan Countries:
Bénin (F.Ahlonsou, L. Anani, G. Atinkpinda, E. Lafia, R.Totongnon), Burkina Faso (S. Coulibaly, H.Dahourou, S. Diallo, P. Madingar, Y. Domo, Z. Hermann, I. Kabore, A. Kiba Koumare, Y. Kientega, Y. Nebie, S. Ouattara, M. Sanou, S. Sawadogo), Burundi (P. Bizimana, L. Ndorere, D. Nzosaba), Cameroon (D. Mbanya, M. Mbangue, S. Mole, J.B. Tapko, C. Tayou Tagny, M. Toukam, S Ewodo, E Guekeng, F Ngo Sack), Comores (H. Said Fazul), Congo- Brazzaville (A. Bokilo, G. Boukatou, A. Dokekias, F. Dolama, J.P. Elenga), Democratic Republic of Congo (S. Agasa, J.M. Bayongwa, Maheshe, H. Kabulo, C. Kakema, S. Kapinga, R. Kazadi, S. Kiyombo Nsenga, B. Malenga Nkanga, P. Mampasi, G. Mayuku, M. Mbelu Kalonji, G.O. Mbensa, P. Misingui, L. Mukendi, D. Mulapi-Tira, D. Ndakala, J.P. Ndjulm Olish, M. Nganga Nkanga, C. Ngandu Bwangandu, S. Tutala Kinkela, S. Y. Ramazani, E. Kabu) Gabon (L. Kouegnigan, J. Ndong, S. Obame, M. Ntsame), Guinea (A. Tata Baldé), Ivory Coast (A. Achy Brou, M. Diané Kouao, J. Hyda, S. Konaté, J.C. Kpangni, A. Nguessan, R. Nguessan- Blao, M. Sika, D. Sawadogo, J. Tchimou, S. Yassonguin, Y Coulibaly, T Bierou, JC Kpangni, M Asso Sika, S Sissoko, D Mankoua),Madagascar (F. Herisoa, J. Holianjavony, P. Hanitriniala, A. Rakoto Alson, R. Rasoarimalalanarivo, M. Razafimahefa, N Andriambelo), Mali (A. Ba, K. Bouréma, M. Cissé, A. Diarra, M. Fomba, G.Y. Gakou, S. Guindo, H. Guitteye, B. Mounirou, S. Oumar Coulibaly, C. Tièman, H. Touré), Mauritania (B. Ciré Ba, M. Abdallahi ould Boullahi), Niger (H Hamissou, A Mahaman, A Brah Maman, A Boulama, B Dan Lélé, R. Yahaha, A. Brah, K.R. Diallo, S. Hadiza, A. Mahane Kabirou, Z. Moussa Mayaki), Rwanda (S Gatare), Sénégal (T.A. Diallo, S. Diop, T. Ndiaye Dieye, M. Gadji, Y. Guèye, M. Seck, A.B. Senghor, D. Thiam, A.O. Touré), Tchad (D. Mbanga, Z. Soureya), Togo (E.A. Akueté, E. Awitala, L. Feteke, H. Magnang, K. Mawussi, Y. Ségbéna, A. Vovor).
Maghreb countries:
Algeria (K. Kezzal), Morocco (A. Abouyoub, M. Benajiba, N. Benchemsi, K. Hajjout, K. Lahjouji, H. Othmani), Tunisia (I. Ben Amor, J. Gargouri, N. Mojaat Moula, H. Skouri).
International collaborators:
The University of California, San Francisco & Blood Systems Research Institute of San Francisco (E. Murphy, C. Shiboski), Institut national de la transfusion sanguine, Paris (S. Laperche), World Health Organization (A. Loua).
Footnotes
Competing interests: the authors have no competing interests.
References
- 1.Bates I, Chapotera G, McKew S, et al. Maternal mortality in sub-Saharan Africa: the contribution of ineffective blood transfusion services. Bjog 2008;115:1331–1339. [DOI] [PubMed] [Google Scholar]
- 2.Lefrère JJ, Dahourouh H, Dokekias AE, et al. Estimate of the residual risk of transfusion-transmitted human immunodeficiency virus infection in sub-Saharan Africa: a multinational collaborative study. Transfusion 2011;51(3):486–92. [DOI] [PubMed] [Google Scholar]
- 3.Custer B, Zou S, Glynn SA, Makani J, et al. Addressing gaps in international blood availability and transfusion safety in low- and middle-income countries: a NHLBI workshop. Transfusion 2018. May;58(5):1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tagny CT, Diarra A, Yahaya R, et al. Characteristics of blood donors and donated blood in sub-Saharan Francophone Africa. Transfusion 2009;49:1592–9. [DOI] [PubMed] [Google Scholar]
- 5.Tagny CT, Diané M, Touré H, et al. Transfusion safety in Francophone African Countries: an Analysis of Strategies for the Medical Selection of Blood Donors. Transfusion 2012; 52(1):134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laperche S, Boukatou G, Kouegnigan L, et al. Transfusion safety on the African continent: an international quality control of virus testing in blood banks. Transfusion 2009;49(8):1600–8. [DOI] [PubMed] [Google Scholar]
- 7.Laperche S Francophone African Group for Research in Blood Transfusion. Multinational assessment of blood-borne virus testing and transfusion safety on the African continent. Transfusion 2013;53(4):816–26. [DOI] [PubMed] [Google Scholar]
- 8.Tagny CT, Murphy EL, Lefrère JJ, et al. The Francophone Africa Blood Transfusion Research Network: a five-year report (2007–2012). Transfus Med 2013;23(6):442–4. [DOI] [PubMed] [Google Scholar]
- 9.Services for Disease Control and Prevention. Progress Toward Strengthening National Blood Transfusion Services - 14 Countries, 20082010 Morbidity and Mortality Weekly Report MMWR 2011; 60(46):1578–1582. [PubMed] [Google Scholar]
- 10.WHO African region current status on blood safety and availability in the WHO African Region — Report of the 2013 survey World health organization regional office for Africa, Brazzaville, 2017. [Google Scholar]
- 11.Katz LM, Donnelly JJ, Gresens CJ, et al. Report of a workshop on ensuring sustainable access to safe blood in developing countries: International Blood Safety Forum, March 24, 2017. Transfusion 2018. May;58(5):1299–1306. [DOI] [PubMed] [Google Scholar]
- 12.Minga A, Dohoun L, Abo Y, et al. Risk behaviors in volunteer blood donors who seroconverte [DOI] [PubMed]
- 13.Tagny CT, Nguefack-Tsague G, Fopa D, et al. Risk factors for human immunodeficiency virus among blood donors in Cameroon: evidence for the design of an Africa-specific donor history questionnaire. Transfusion 2017;57(8):1912–21 [DOI] [PubMed] [Google Scholar]
- 14.Aneke JC, Okocha CE .Blood transfusion safety; current status and challenges in Nigeria. Asian J Transfus Sci. 2017;11(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DJ, Field S, Delaney M, et al. Problems and Approaches for Blood Transfusion in the Developing Countries. Hematol Oncol Clin North Am. 2016;30(2):477–95. [DOI] [PubMed] [Google Scholar]
- 16.Tagny CT, Kapamba G, Diarra A, et al. The training in blood transfusion is still insufficient in the blood services of Francophone sub-Saharan Africa: results of a preliminary study. Transf.Clin.Biol 2011;18(5–6):536–41 [DOI] [PubMed] [Google Scholar]
- 17.Gallaher J, Mulima G, Kopp D, et al. Consequences of centralized blood bank policies in sub-Saharan Africa. The Lancet Global Health 2017;5(2):131–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slopecki A, Smith K, Moore S. The value of Good Manufacturing Practice to a Blood Service in managing the delivery of quality. Vox Sang 2007;92(3):187–96. [DOI] [PubMed] [Google Scholar]
- 19.Pereira P, Westgard JO, Encarnação P, et al. Quality management in European screening laboratories in blood establishments: A view of current approaches and trends. Transfus Apher Sci. 2015;52(2):245–51. [DOI] [PubMed] [Google Scholar]
- 20.Kabinda JM, Miyanga SA, Misingi P, et al. Hepatitis B and C among volunteer non-remunerated blood donors in Eastern Democratic Republic of Congo. Transfus Clin Biol. 2014;21(3):111–5. [DOI] [PubMed] [Google Scholar]