Abstract
Background
South Asians (SAs) experience a disproportionate burden of high blood pressure (BP) in the US, arguably the most preventable risk factor for cardiovascular disease.
Objective
We report 12-month results of an electronic health record (EHR)-based intervention, as a component of a larger project, “IMPACT”. The EHR intervention included launching hypertension patient registries and implementing culturally-tailored alerts and order sets to improve hypertension control among patients treated in 14 New York City practices located in predominantly SA immigrant neighborhoods.
Design
Using a modified stepped wedge quasi-experimental study design, practice-level EHR data were extracted, and individual-level data were obtained on a subset of patients insured by a Medicaid insurer via their data warehouse. The primary aggregate outcome was change in proportion of hypertensive patients with controlled BP; individual-level outcomes included average systolic BP (SBP) and diastolic BP (DBP) at last clinic visit. Qualitative interviews were conducted to assess intervention feasibility.
Measures
Hypertension was defined as having at least one hypertension ICD-9/10 code. Well-controlled hypertension was defined as SBP<140 mmHg and DBP<90 mmHg.
Results
Post-intervention, we observed a significant improvement in hypertension control at the practice level, adjusting for age and sex patient composition (aRR: 1.09, 95% CI: 1.04–1.14). Among the subset of Medicaid patients, we observed a significant reduction in average SBP and DBP adjusting for time, age, and sex, by 1.71 mmHg and 1.13 mmHg, respectively (p<0.05). Providers reported feeling supported and satisfied with EHR components.
Conclusions
EHR initiatives in practices serving immigrants and minorities may enhance practice capabilities to improve hypertension control.
Keywords: Health information technology (HIT), Electronic health record (EHR), Community-clinical linkages, Hypertension, Million Hearts® Initiative, Immigrant Health, South Asians
Introduction
Cardiovascular disease (CVD) is the leading cause of death in the United States (US). Among risk factors known to contribute to CVD, high blood pressure (BP) is arguably the most relevant and preventable, accounting for nearly half of all CVD events.1–4 Nationwide, approximately 30.3% of adults live with hypertension.2
In a nationwide effort to reduce hypertension burden, the Centers for Disease Control and Prevention (CDC) partnered with the Centers for Medicare and Medicaid Services (CMS) to launch the Million Hearts® Initiative in 2011, with the goal to reduce 1 million heart attacks and strokes every five years.5 The Million Hearts initiative encourages innovative approaches to improve CVD risk factors – in particular, BP control,5 with a focus on team-based health care, implementation of patient-centered medical homes (PCMH) to support coordinated care, and the utilization of health information technology (HIT).5 In 2013, the Community Preventive Services Task Force (CPSTF) called for the use of clinical decision-support systems (CDSS) for the prevention of CVD.6, 7 CDSS are a form of HIT, frequently embedded within electronic health record (EHR) systems, designed to assist providers in clinician decision making at the point of care.6–8 Examples of CDSS are registry reports for identifying at-risk patients, reminders for overdue BP screenings, recommendations for health behavior changes, and alerts when indicators for CVD risk factors are not at goal.6
Despite national recommendations, to date there have only been a limited number of HIT-based interventions targeting practices serving immigrant and/or minority communities, leaving these communities “under the radar” of national initiatives promoting EHR-based solutions through incentive streams.9–12 Unfortunately, residents in those communities are often the most vulnerable to disparities in hypertension prevalence and management. For example, in New York City (NYC), where a significant proportion of immigrant communities, including South Asians (SAs), seek care in small community-based practices,13 the age-adjusted prevalence of hypertension is 27.5% for non-Hispanic white adults but 43.0% among SA adults, according to the 2013–2014 NYC Health and Nutrition Examination Survey (NYC HANES).14
It is in this context that Project IMPACT (Implementing Million Hearts for Provider and Community Transformation) was initiated.15,16 Project IMPACT is a CDC-funded 5-year modified stepped wedge quasi-experimental study designed to test the feasibility, adoption, and impact of integrating a multi-level intervention to improve hypertension control among patients in small NYC practices located in neighborhoods with a large proportion of SA residents, involving: 1) a practice and provider-level EHR-based intervention, and 2) a community health worker (CHW)-led health coaching intervention.16, 17 The current study characterizes the feasibility and effect of the EHR component of our multi-level intervention by examining change in practice-level and individual-level patient BP control between baseline and at least one-year post-intervention implementation.
Methods
Partnerships and Recruitment of Practices
Aligning with strategies promoted through the Million Hearts® Initiative,5 Project IMPACT was led by the New York University-City University of New York Prevention Research Center (NYU-CUNY PRC) in collaboration with NYC-based not-for-profit health insurance company, Healthfirst (HF), and Island Peer Review Organization (IPRO), a quality improvement organization of New York State. The intervention study staggered an EHR and an integrated EHR-CHW intervention using a modified stepped wedge design.16 Five non-randomized clusters of 2–4 practices each implemented the intervention, for a total of 16 enrolled practices located in largely SA neighborhoods in NYC. Four of the 16 practices had identical patients linked to two physicians, so analyses were combined to present results for 14 practices. To be eligible for enrollment into Project IMPACT, independent practices had to be part of HF’s network and identify as having at least 70% of their patient population be SA, per self-reported by the lead physician.16 Practices were required to have an operating EHR, specifically eClinicalWorks (eCW)18 or MDLand19, for at least 12 months prior to enrollment into the study.16, 18, 19 A total of 133 practices were initially approached for outreach in partnership with HF by generating a list of practices in high density SA neighborhoods. Of these, 25.6% (n = 34) completed the practice survey, and 12% (n=16) enrolled (Figure 1). The EHR component of the intervention launched in January 2016 in the first round of practices and the remaining four rounds implemented the intervention at phased ~3-month intervals thereafter.
Figure 1.
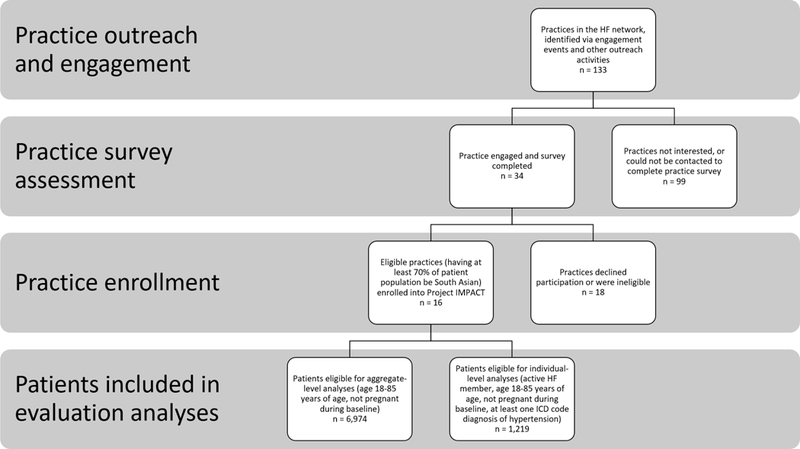
Practice- and individual-level consort diagram for evaluation of Project IMPACT’s Electronic Health Record (EHR) intervention
Intervention Design
The EHR intervention implementation consisted of a two-day training for providers and staff on: 1) generating hypertension patient registry reports; and 2) developing and implementing medical alerts and order sets (Table 1).16, 20 Training on patient registries focused on generating routine reports to identify patients with diagnosed hypertension with an uncontrolled BP reading at last clinic visit to prioritize follow-up visits and identify patients lost-to-follow up. Medical alerts prompted providers to enter BP measurements in standardized fields, repeat measures for patients with uncontrolled BP, and create follow-up appointments for hypertensive patients dependent on BP control status. Alerts functioned as a reminder system and call-to-action within the EHR. In the two EHR systems in which our intervention was implemented, the end-user could bypass these manually in any encounter, though all trainees were advised to acknowledge and follow the function. Order sets included prescriptions, lab tests, and counseling orders “pre-set” for patients with hypertension which were linked to evidence-based, culturally tailored, in-language educational materials tailored to the SA patient population.16 Hypertension was defined as having at least one ICD-9 or 10 code for hypertension during the baseline year. Because the intervention was launched in 2016, uncontrolled BP was defined using the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7) guidelines, having a systolic BP (SBP) of ≥140 mmHg or diastolic BP (DBP) level of ≥90 mmHg.21
Table 1.
Electronic health record (EHR) intervention components
| Component | Definition | Specification | Purpose |
|---|---|---|---|
| Registry reports | Feature within the EHR that allows providers to query and group information on their patients based on specified criteria, ranging from diagnoses of particular health conditions to demographic characteristics | Registry reports were built to create lists of hypertension patients, by control status | To help practices plan and prioritize patient visits and assist with scheduling appointments |
| Alerts | Patient-specific or “global” feature that applies to any patient that satisfies specified criteria | Alerts were tailored to trigger when a blood pressure measurement is missing for the patient at the point of care, and prompts the provider for a repeat measure if elevated, and to create an appointment for follow-up | To help providers with the prevention, identification, and management of hypertension |
| Order sets | Standardized sets of evidence-based treatment guidelines that apply when linked to an alert | Order sets were built to include a combination of prescriptions, lab tests, and counseling orders “pre-set” for patients with hypertension. Within the counseling orders, evidence-based and culturally tailored in-language educational materials are uploaded for distribution to patients fluent in a variety of South Asian languages |
Additional components included in the intervention were the activation of standardized and mandatory fields within the EHR (ex: BP fields), standardizing race/ethnicity subgroup documentation, and training on codes that may be used to bill for the time spent counseling patients.16 Practices were also encouraged to consider applying for Meaningful Use (MU)/PCMH recognition status, and IPRO offered services to help practices improve their documentation and reporting processes to achieve these goals. After implementation, practices participated in seven on-site technical assistance (TA) sessions over the next year to answer questions and assess adherence to the intervention protocol. Ad hoc sessions were scheduled as necessary to reinforce the trainings, resolve technical issues, and answer remaining questions. A formal start date was assigned for each round of practices, after all practices had completed training, to group practices with similar implementation training dates together. Additional details of Project IMPACT’s study design and protocol were previously published.16
Data Sources and Study Outcomes
Eligibility criteria for evaluation analyses included age being 18–85 years, having at least one diagnosis of hypertension at a clinic visit and not pregnant during the baseline year, 12-month pre-intervention implementation (Figure 1). Data extraction covered patient visits occurring from baseline to at least 12-months post-intervention implementation.
Aggregate Practice-Level Data
One week prior to training, Project IMPACT staff assessed practice baseline workflow, staff capacity, and use of the EHR with ethnographic observation, and practice- and provider-level baseline surveys.16 Practice staff were also asked to complete a training evaluation survey immediately post-training to assess satisfaction with the training format and content. One-year after practices implemented the EHR intervention, semi-structured interviews were conducted with providers and staff at 13 sites to discuss acceptability and feasibility of the intervention.
To evaluate the effect of the EHR intervention components, practice-level EHR data were extracted every six months by study staff using EHR registry report tools and customized reports. The primary practice-level outcome was change in proportion of hypertensive patients with controlled BP.
Reporting differences between the two EHR systems resulted in minor differences in how the primary outcome was operationalized for practice-level analyses. For clinics using MDLand, the outcome denominator included all active patients diagnosed with hypertension over that period. For clinics using eCW, the outcome denominator included all active patients diagnosed with hypertension and who had a BP reading at their last visit within the period. To evaluate the impact of this difference, we conducted a manual exploration by applying the MDLand rules to a small subset of patients at two clinics using eCW. The aggregate outcome measures (changes in pre- and post-proportions) resulting from the eCW and MDLand definitions differed by less than 1% (data not shown).
Individual Patient-level Data
To evaluate the effect of the EHR intervention at the individual-level, EHR and HF claims data were extracted by HF for the subset of hypertensive patients with HF insurance and de-identified. Elements extracted from the EHR included: month/year dates of office visits with accompanying SBP and DBP and body mass index (BMI) measurements. Elements extracted through the HF claims data warehouse included age, gender, race/ethnicity, language, and select health conditions: diabetes (ICD-9: 250.*; ICD-10: E10.*, E11.*) and hypercholesteremia (ICD-9: 272.*; ICD-10: E78.*). EHR- and claims-based data were merged via a unique patient ID. The main individual-level outcome for this study was average SBP and DBP at last clinic visit. A secondary individual-level outcome was odds of controlled BP at last visit among hypertensive patients.
Data Analyses
Practice-Level
We summarized practice characteristics using data from the baseline surveys and ethnographic observations. The age and sex distribution of patients was characterized using aggregate data. Generalized Poisson mixed effects models (crude and adjusted for age and sex) tested the effects of the intervention on hypertension control, assuming a linear effect over time, along with models that relaxed the linearity assumption. We summarized PCMH/MU recognitions achieved across the sites. Fidelity to intervention was summarized by type, frequency, and utilization of registry reports, alerts, and order sets per practice. Interviews with providers and clinic staff were transcribed where audio-recorded. Interview notes and transcripts were thematically analyzed using a mix of inductive and deductive approaches.
Patient-level
Demographics, BMI, and common co-morbidities were summarized among active HF hypertensive patients using individual-level data. Pearson chi-square test statistics were calculated to examine differences in the proportion of HF patients with well-controlled versus uncontrolled hypertension during the baseline year. A linear mixed-effect model was run to measure the effect of the intervention on average SBP and DBP. A secondary logistic mixed-effect model was run to measure the intervention effect on the odds of hypertension control. All models included a random intercept component, accounting for patients nested by site, adjusting for age, sex and time. Race/ethnicity was initially considered as a covariate in the models, but after adjustment, estimates did not change and was thus removed.
For both aggregate- and individual-level analyses, time was divided into six-month periods, beginning at baseline, following the stepped wedge design and data collection efforts. Statistical significance was accepted at p < 0.05. Analyses were completed using SAS 9.4 and R Version 1.1.414.
Results
Baseline practice characteristics
At enrollment, participating practices reported having been operational for an average of seven years, treating an average of 178 patients a week, with most patient care revenue coming from Medicaid (73.4%), data not shown. By comparison, eligible practices that opted to not enroll in the intervention had been operating longer (average 12 years) and were larger (average of 665 patients a week). On average, participating practices had two full-time and two part-time physicians, and four full-time and three part-time staff. Most patients in enrolled practices (75%) identified as SA, per lead provider self-report.
According to baseline provider-level surveys, no providers were familiar with the Million Hearts® Initiative. Ethnographic observation identified that no practices routinely or appropriately utilized any of the intervention components prior to implementation. Half (n=7 practices) were not consistently or appropriately documenting language and/or race/ethnicity categories in the EHR. While all practices had English-language patient education materials available in the waiting room, most did not have translated materials for their non-English speaking patients. For practices relying heavily on walk-in patients (n=5 practices), staff reported this affected their ability to perform patient follow-up to prioritize high-risk patients and led to longer wait times. Staff at all practices reported not performing reminder phone calls, and all routinely double-booked patients, expecting that most would be no-shows and that walk-in patients would fill the time slot. In some cases, patients were observed leaving the office immediately after seeing the provider without checking-out or making a follow-up appointment.
As shown in Table 2, there were 6,974 active hypertensive patients across all 14 sites at baseline. Prior to intervention implementation, 61.9% of all hypertensive patients had well-controlled BP. There was no substantial variability in the prevalence of controlled BP across demographic subgroups. In the subgroup of HF patients (n=1,219), 71.2% were considered well-controlled at last clinic visit at baseline. Statistically significant differences in the proportion controlled between sex was observed among HF patients (75.2% and 67.7% of females and males, respectively, p<0.01).
Table 2.
Characteristics of hypertensive patients at baseline# at all participating practices (n=14) and among the subset of hypertensive patients with Healthfirst (HF) insurance
| All Patients | HF-insured Patients | |||||
|---|---|---|---|---|---|---|
| Total patients with hypertension | Proportion of patients well-controlleda | Total HF patients with hypertension | Proportion of patients well-controlleda | |||
| n | n | % | n | n | % | |
| 6974 | 4320 | 61.9% | 1219 | 868 | 71.2% | |
| Demographics | ||||||
| Sex | ||||||
| Male | 3722 | 2307 | 62.0% | 650 | 440 | 67.7%* |
| Female | 3252 | 2013 | 61.9% | 569 | 428 | 75.2% |
| Age | ||||||
| 18–39 | 619 | 342 | 55.3% | 92 | 65 | 70.7% |
| 40–59 | 3474 | 2179 | 62.7% | 532 | 397 | 74.6% |
| 60–85 | 2881 | 1799 | 62.4% | 595 | 406 | 68.2% |
| Race/Ethnicity | ||||||
| Non-Hispanic White | - | - | - | 24 | 17 | 70.8% |
| Black or African American | - | - | - | 110 | 73 | 66.4% |
| Asian or Pacific Islander | - | - | - | 342 | 259 | 75.7% |
| Hispanic | - | - | - | 54 | 39 | 72.2% |
| Other | - | - | - | 689 | 480 | 69.7% |
| Language | ||||||
| English | - | - | - | 1044 | 746 | 71.5% |
| Non-English | - | - | - | 175 | 122 | 69.7% |
| Body Mass Index (BMI) | ||||||
| <18.5–24.9 Underweight, Normal | - | - | - | 319 | 240 | 75.2% |
| 25.0 – 29.9 Overweight | - | - | - | 543 | 388 | 71.5% |
| >=30.0 Obese | - | - | - | 345 | 232 | 67.3% |
| Health conditions | ||||||
| Hypercholesteremia | - | - | - | 893 | 632 | 70.8% |
| Diabetes | - | - | - | 709 | 498 | 72.4% |
practice-level and individual-level baseline time period constitutes 6- and 12-months prior to the group start date, respectively
well-controlled defined as systolic <140 mmHg and diastolic <90 mmHg at last blood pressure measurement
p<0.01 compares demographics and health conditions by control status among HF patients
Effect of Intervention on Blood Pressure Outcomes
At the practice-level, hypertension control rates showed slight improvements in hypertension control post-intervention (RR: 1.09, 95% CI: 1.04–1.14) after adjusting for age and sex. However, the alternative model relaxing the linearity assumption, despite a better fit, did not estimate a clear relationship (RR: 1.03, 95% CI: 0.98, 1.09). At the individual level among HF patients, we observed a statistically significant effect of the intervention on average SBP, adjusting for time, age, and sex, with a decrease of average SBP and DBP of 1.71 mmHg and 1.13, respectively, (p<0.05) [Table 3]. Similar to the practice-level findings, the proportion of HF patients with well-controlled BP improved after adjusting for demographics (aOR: 1.36, p<0.05).
Table 3.
EHR intervention patient-level effect on average systolic and diastolic blood pressure (BP) and proportion controlled
| Outcome | Controlᵻ | Interventionᵻ | Overall treatment effect on average BP | p-value* |
|---|---|---|---|---|
| Systolic BP | −1.71 (−2.88, −0.54) | 0.004 | ||
| Time 1 | 129.1 (128.3, 129.9) | − | ||
| Time 2 | 131.6 (130.7, 132.6) | − | ||
| Time 3 | 128.5 (127.3, 129.7) | 127.7 (126.2, 129.3) | ||
| Time 4 | 130.4 (129.1, 131.7) | 130.3 (129.0, 131.6) | ||
| Time 5 | − | 128.0 (127.0, 128.9) | ||
| Time 6 | − | 132.2 (130.7, 133.6) | ||
| Diastolic BP | −1.13 (−1.89, −0.37) | 0.004 | ||
| Time 1 | 78.7 (78.2, 79.2) | − | ||
| Time 2 | 79.5 (78.9, 80.0) | − | ||
| Time 3 | 78.1 (77.4, 78.8) | 78.1 (77.3, 79.0) | ||
| Time 4 | 79.0 (78.2, 79.8) | 78.4 (77.6, 79.2) | ||
| Time 5 | − | 76.8 (76.0, 77.5) | ||
| Time 6 | − | 78.6 (77.5, 79.6) | ||
| Overall treatment odds of BP control | ||||
| Controlled hypertension | 1.36 (1.08, 1.71) | 0.009 | ||
| Time 1 | 73.0% (70.6, 75.4) | − | ||
| Time 2 | 67.7% (65.1, 70.3) | − | ||
| Time 3 | 72.9% (69.6, 76.1) | 75.5% (71.6, 79.4) | ||
| Time 4 | 70.2% (66.4, 74.0) | 73.6% (69.8, 77.4) | ||
| Time 5 | − | 78.3% (75.8, 80.9) | ||
| Time 6 | − | 68.3% (64.3, 72.2) |
For systolic and diastolic BP outcomes: average BP, 95% CI; For controlled hypertension: proportion controlled, 95% CI
models adjusted for age, sex, and time
Acceptability, feasibility, and fidelity
Twelve of 14 practices completed the training evaluation survey; all practices’ lead physician and/or manager agreed or strongly agreed they felt prepared to use the patient registry to improve appointment adherence and prioritize uncontrolled hypertensive patients on a weekly basis; all practices strongly agreed or agreed they were prepared to use point-of-care alerts to improve hypertension practices, and 11 strongly agreed or agreed they were prepared to use order sets to improve hypertension management.
One-year post-implementation, themes from semi-structured interviews with practice staff illuminated satisfaction with the EHR intervention components. Interviewees described that registry reports helped them to reliably see which of their patients had uncontrolled hypertension and prompted them to call patients to schedule a clinic visit. Staff noted that alerts and order sets were “easy to implement” into their EHR system, and that the EHR components had improved their ability to monitor and serve at-risk patients and advanced their efforts to obtain PCMH certification. When reflecting on barriers to integrating EHR components, providers reported that limited time and high staff turnover slowed technical advancement to navigate new features within an EHR system.
Less than two years post-launch of the intervention, 6 of the 14 practices achieved MU recognition, and 4 achieved PCMH Level 3 recognition. Regarding fidelity to intervention, all practices enrolled into Project IMPACT had utilized all components of the EHR intervention. Though alerts can be manually bypassed for specific patient encounters, these remained globally activated at each site during follow-up fidelity assessments. The frequency of use of particular components varied by practices; nearly two years post-implementation, practices with MDLand EHR systems scheduled appointments with hypertensive patients more often than eCW-based practices (MDLand sites averaged 11 reports developed; eCW sites averaged 4), and more frequently tracked uncontrolled hypertensive patients (MDLand sites averaged 7 reports developed; eCW averaged 4). Eleven of 14 practices completed all 7 TA sessions.
Discussion
To our knowledge, this is the first study to investigate the impact of practice-level quality improvement efforts for BP control among community-based practices serving the SA patient population, which faces significant disparities in CVD. At baseline, these practices were not using the EHR for population health management of their hypertensive patients, consistent with prior observations that small primary care practices are largely missed by larger municipal quality improvement efforts.13 This is further supported by our finding that the average level of BP control across our group of practices was 61.9% at baseline, lower than other rates of BP control reported among particular racial and ethnic groups in NYC.22
Our results demonstrate that the EHR intervention significantly improved practice-level hypertension control (8%), a level similar to other published studies that report average improvements in BP control ranging from 9–18%.23 Additionally, patient-level average decrease in SBP and DBP is consistent with literature where similar EHR intervention studies report average improvements in blood pressure.24, 25 Though we found a smaller change in magnitude in SBP and DBP (1.71 mmHg and 1.13 mmHg decrease, respectively) compared to other similar studies (5.2 SBP and 6.0 DBP26; 21.7 DBP27; 4.6 SBP28), these studies had smaller sample sizes, shown to produce greater effect sizes.24 Importantly, most studies have been conducted in federally qualified health center or larger health systems with more resources to support ongoing HIT efforts.24, 28, 29
Though the intervention demonstrated practice-level effects on BP control, findings should be interpreted with caution. As demonstrated in Figure 2, there was substantial variability in BP control trends across practices, which may have several explanations. Our fidelity assessment demonstrated that although all practices used the alert/order set functions post-intervention, there was variability in the generation and use of hypertension registries. We believe this is a result of workflow and operational issues; though staff were trained to save individual reports each time reports are generated, practices may have underreported their use of registry reports, especially as they became used to incorporating the saving of reports into their workflow. Additionally, MDLand automatically saves these reports, whereas eCW requires an extra manual step to save registry reports, which may explain the more favorable fidelity results from MDLand users.18, 19 Relatedly, practices reported high staff turnover in these settings and employ a significant number of part-time staff who may not have been part of the EHR intervention training. We encouraged individuals who received intervention training to train new staff to mitigate this issue. Finally, because this intervention was designed to serve the needs of low-resource practices, practices were initially trained to generate registry lists for uncontrolled hypertensive patients using standard “out-of-the-box” EHR tools. However, in both EHR platforms, the process for generating such lists with standard tools entails manual procedures that are time-consuming. For this reason, practices were offered additional software upgrades and installments to facilitate the process of generating these registry lists after intervention implementation. Future analysis will assess the impact of using these enhanced tools on improvements in practice-level BP control.
Figure 2.
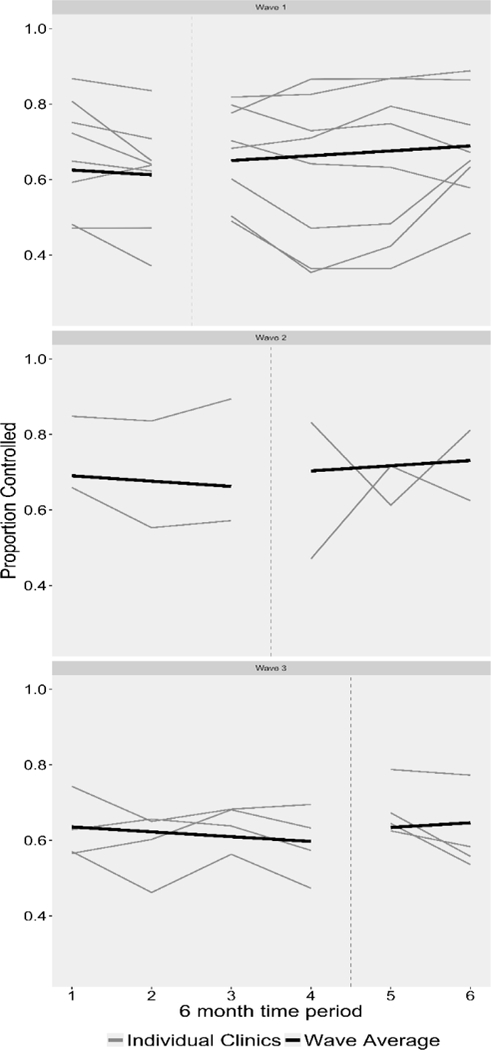
Change in Practice-level Proportion of Hypertensive Patients with Well-Controlled Blood Pressure (BP) from Pre- to Post-Intervention Implementation
Other explanations may include a limited capacity to integrate EHR intervention components into practice workflow; some practices may not have sustained activities after TA sessions ended. Practices with lower fidelity to intervention may experience less robust or negative improvements in BP control, particularly prior to the integration of the CHW intervention. Additionally, an alternative practice-level model relaxing the linearity assumption had a better fit, but did not produce a statistically significant effect of the intervention on practice-level hypertension control, which may in part reflect high variability in BP control across practices. However, given that practices received ongoing technical support to strengthen intervention-related activities, we believe that the linearity assumption may be appropriate.
Limitations
There are several limitations to this study. First, the study utilized a non-randomized design, where sites were allocated by convenience to various waves of the stepped-wedge study design in group of 3–4 practices. This modification was made due to an inability to recruit all study practices at the start of the study. We are thus unable to account for unmeasured systematic differences between intervention and comparison periods which may impact results. Another limitation is that minor differences in data collection for our primary outcome variable were introduced due to EHR vendor differences. However, a sensitivity analysis suggested that the slight discrepancy in reporting did not affect results. Also, EHR individual-level data on HF patients were collected on patients that met the eligibility criteria during the baseline year. Patients who changed insurers or were newly insured after 2015 were not included in the analysis; however, because patients who are consistently seen likely exhibited sustained BP improvement, new, uncontrolled hypertensive patients would have strengthened effect estimates. This limitation most likely biases our results towards the null, strengthening our confidence in our results. Finally, point-of-care alerts could be manually bypassed without completing the suggested actions. However, this limitation likely made minimal changes to the overall results being that the global ‘activation’ of these alerts were present in all practices throughout post-implementation.
Conclusion
Our results demonstrate that HIT initiatives and the use of an EHR system enhances practice capabilities to improve hypertension control in settings that serve immigrant dn minority populations with disproportionate health disparities. Our partnership with a payer organization provides a model for engaging small practices serving vulnerable populations in quality improvement efforts. This may be especially relevant for community-based providers facing financial pressures in the context of healthcare reform efforts that prioritize large healthcare systems. As Million Hearts® efforts continue with a focus on improving BP control through the promotion of innovative partnerships, community-clinical linkages, and HIT, EHR-based interventions targeting small practices can contribute to the effort to eliminate CVD disparities.
Acknowledgements
We would like to acknowledge and thank Ahmad Masoud from iRCM, Joanne Vanterpool, MBA and Susan Hollander, MPH, CPHQ, from IPRO, the federally-funded Medicare Quality Innovation Network-Quality Improvement Organization, for their substantial contributions to the implementation of the EHR intervention and their review of key EHR components in this manuscript. We would also like to thank Md Jalal Uddin, Shree Subedi, Sidra Zafar, Mursheda Ahmed, and Mamnunul Haq, the community health workers that contributed to the creation and implementation of the intervention.
Funding
The funding source: This study is supported by grant number U48DP005008 from the Centers for Disease Control and Prevention (CDC). Author’s contributions are additionally supported in part by grant number U58DP005621 from the Centers for Disease Control and Prevention (CDC), P60MD000538, U54MD000538–15, R01DK110048–01A1, UL1TR001445 from the National Institutes of Health (NIH). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and CDC.
Footnotes
Authorship
The authors declare that there are no conflicts of interest.
References
- 1.Fryar CDC T; Li X Prevalence of uncontrolled risk factors for cardiovascular disese: United States, 1999–2010. National Center for Health Statistics; 2012. [Google Scholar]
- 2.Valderrama ALG C; King SC; George MG; Hong Y; Gregg E Vital signs: awareness and treatment of uncontrolled hypertension among adults--United States, 2003–2010. MMWR Morbidity and mortality weekly report. 2012;61(35):703–709. [PubMed] [Google Scholar]
- 3.Pirani N, Khiavi FF. Population Attributable Fraction for Cardiovascular Diseases Risk Factors in Selected Countries: A comparative study. Materia Socio-Medica. 2017;29(1):35–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SA, Winkel M, Ali MK, et al. Cardiovascular mortality associated with 5 leading risk factors: national and state preventable fractions estimated from survey data. Ann Intern Med. 2015;163(4):245–253. [DOI] [PubMed] [Google Scholar]
- 5.Frieden TR, Berwick DM. The “Million Hearts” Initiative — Preventing Heart Attacks and Strokes. New England Journal of Medicine. 2011;365(13):e27. [DOI] [PubMed] [Google Scholar]
- 6.Guide TC. Cardiovascular Disease: Clinical Decision-Support Systems (CDSS) Available: https://www.thecommunityguide.org/findings/cardiovascular-disease-clinical-decision-support-systems-cdss July, 2017.
- 7.Guide TC. Cardiovascular disease: Clinical decision-support systems 2017. [Google Scholar]
- 8.Berner ES, La Lande TJ. Overview of Clinical Decision Support Systems In Berner ES, (Ed). Clinical Decision Support Systems: Theory and Practice. Cham: Springer International Publishing; 2016:1–17. [Google Scholar]
- 9.Understanding the Impact of Health IT in Underserved Communities and those with Health Disparities. NORC at the University of Chicago; 2010. [Google Scholar]
- 10.Ash JS, Sittig DF, Wright A, et al. Clinical decision support in small community practice settings: a case study. Journal of the American Medical Informatics Association : JAMIA. 2011;18(6):879–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopal H Evaluation of a Computerized Clinical Decision Support System and EHR-Linked Registry to Improve the Management of Hypertension in a Community Health Center. Primary Care Development Corporation, Inc. [Google Scholar]
- 12.Litvin CB, Hyer JM, Ornstein SM. Use of Clinical Decision Support to Improve Primary Care Identification and Management of Chronic Kidney Disease (CKD). Journal of the American Board of Family Medicine : JABFM. 2016;29(5):604–612. [DOI] [PubMed] [Google Scholar]
- 13.Scherer MW L Community Perspectives on Advanced Primary Care. 2016. [Google Scholar]
- 14.Fei KR-L JS; Ramons M; Islam N; Trinh-Shevrin C; Yi SS; Chernov C; Perlman SE; Thorpe LE Racial and Ethnic Subgroup Disparities in Hypertension Prevalence, New York City Health and Nutrition Examination Survey, 2013–2014. Preventing Chronic Disease. 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NYU-CUNY Prevention Research Center Available: https://med.nyu.edu/prevention-research/projects/project-impact.
- 16.Lopez PM, Zanowiak J, Goldfeld K, et al. Protocol for project IMPACT (improving millions hearts for provider and community transformation): a quasi-experimental evaluation of an integrated electronic health record and community health worker intervention study to improve hypertension management among South Asian patients. BMC Health Services Research. 2017;17:810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Islam NN S; Peretz P; Matiz LA; Hirsch G; Kane E; Collinsworth A; Kangovi S; Godfrey WK; Hyde J; Matos S; Kumar R; Lopez P; Zhong L; Thorpe L; Trinh-Shevrin C Integration of Community Health Workers into Primary Care Health Systems: The Time for New York is Now!. NYU-CUNY Prevention Research Center; 2016. [Google Scholar]
- 18.eClinicalWorks Available: https://www.eclinicalworks.com/. March 29 2017.
- 19.MDLand Available: http://www.mdland.com/. March 29 2017.
- 20.Njie GJ, Proia KK, Thota AB, et al. Clinical Decision Support Systems and Prevention: A Community Guide Cardiovascular Disease Systematic Review. American journal of preventive medicine. 2015;49(5):784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reference card from the seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure (JNC 7). [PubMed]
- 22.Basset MT. Hypertension Toolkit Letter. New York City: Department of Health and Mental Hygiene; 2017. [Google Scholar]
- 23.Using health information systems to identify and control hypertension: Lessons from the ASTHO Million Hearts learning collaborative 2016. [Google Scholar]
- 24.Shojania KGM KM; Wachter RM, Owens DK Closing The Quality Gap: A Critical Analysis of Quality Improvement Strategies. AHRQ Publication; 2005;3. [PubMed] [Google Scholar]
- 25.Hetlevik I, Holmen J, Kruger O. Implementing clinical guidelines in the treatment of hypertension in general practice. Evaluation of patient outcome related to implementation of a computer-based clinical decision support system. Scandinavian journal of primary health care. 1999;17(1):35–40. [DOI] [PubMed] [Google Scholar]
- 26.Friedman RH, Kazis LE, Jette A, et al. A telecommunications system for monitoring and counseling patients with hypertension. Impact on medication adherence and blood pressure control. American journal of hypertension. 1996;9(4 Pt 1):285–292. [DOI] [PubMed] [Google Scholar]
- 27.McAlister NH, Covvey HD, Tong C, et al. Randomised controlled trial of computer assisted management of hypertension in primary care. British medical journal (Clinical research ed). 1986;293(6548):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery AAF T; Peters TJ; MacIntosh C; Sharp DJ Evaluation of Computer Based Clinical Decision Support System and Risk Chart for Management of Hypertension in Primary Care: Randomised Controlled Trial. BMJ. 2000;320(7236):686–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morrow RWF J; Kelly KF; Shea LA; Spence MM; Sullivan JN; Cerniglia JR, Yang Y Improving diabetes outcomes using a web-based registry and interactive education: A multisite collaborative approach. Journal of Continuing Education in the Health Professions. 2013;33(2):136–145. [DOI] [PubMed] [Google Scholar]


