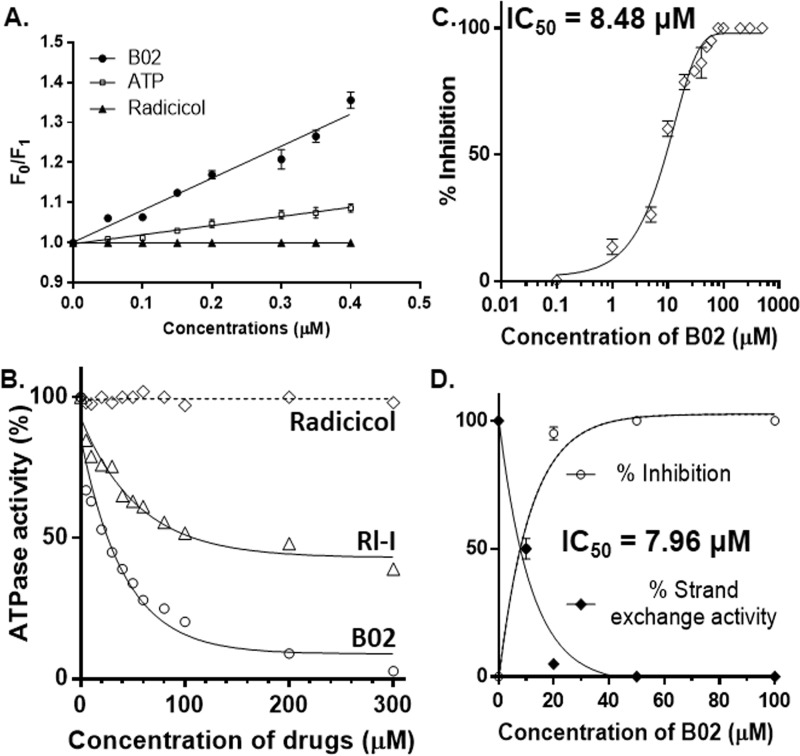
Figure 2.
B02 binds to PfRad51 and inhibits its ATP hydrolysis activity. A, quenching of intrinsic fluorescence of Trp-170 of PfRad51 upon B02 binding. Stern-Volmer plots showing the ratio of intrinsic fluorescence (F0) and quenched fluorescence (F1) at different concentrations of ligand binding are shown. The excitation was at 295 nm, and the emission was recorded at 332 nm. Data are the mean ± S.D. from three experiments. B, ssDNA-dependent ATPase activity of PfRad51 in the presence of various concentrations of drugs (as indicated on the x axis) as indicated. C, at 200 μm ATP concentration and 60 μm ssDNA, the activity of 1 μm PfRad51 is inhibited by B02 with an IC50 value of 8.48 μm. Mean and standard errors from three independent experiments are plotted. D, three-strand exchange activity of PfRad51 is inhibited by B02 with an IC50 value of 7.96 μm. Mean and standard errors from three independent experiments are plotted. LD, linear dsDNA (substrate); NC, nicked circular DNA (product).