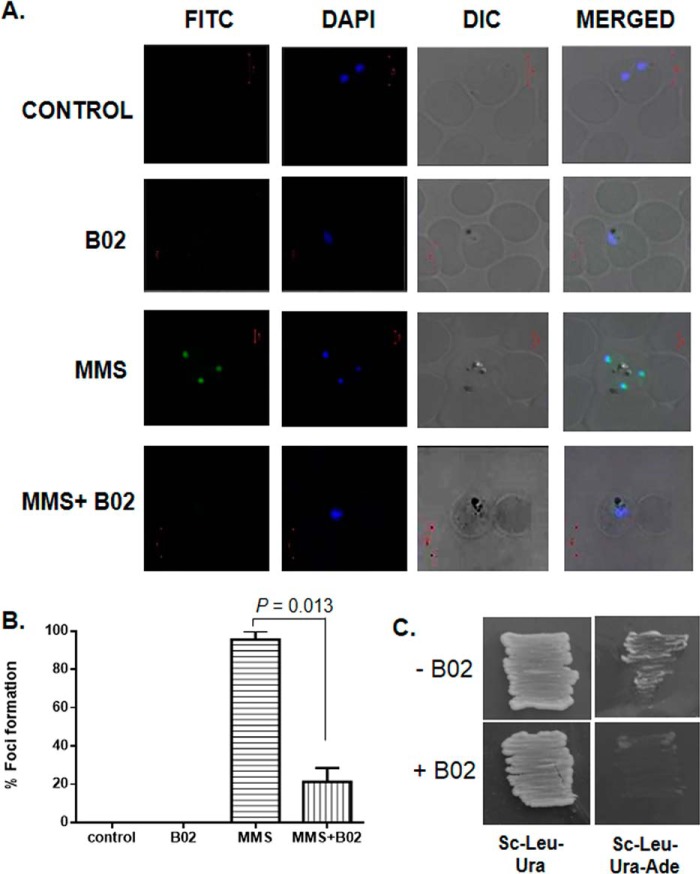
Figure 4.
B02 inhibits the formation of PfRad51 foci upon DNA damage. A, IFA displays PfRad51 foci (FITC) upon treatment with the DNA-damaging agent MMS (3rd row). Such foci are not visible in the control cells (untreated with MMS) neither in the presence (2nd row) nor in the absence of 8 μm B02 (1st row). MMS-induced PfRad51 foci formation are inhibited in the presence of 8 μm B02 (4th row). DAPI staining indicates the location of the parasite nucleus. B, quantitative analysis of PfRad51 foci formation. Percent of focus formation is defined as the number of infected RBC having PfRad51 foci (FITC-stained) of 100 infected RBC (DAPI-stained). The mean and the standard deviations from three independent experiments are plotted. Two-tailed t test was performed to obtain the statistical significance. The p value is indicated at the top. C, self-interaction of PfRad51 is inhibited in the presence of 8 μm B02. pGADC1 and PGBDUC1 are the parent plasmids encoding the GAL4 activation domain and DNA-binding domain, respectively. DNA fragments corresponding to the full-length WT PfRAD51 ORF were fused to the GAL4 activation domain in pGADC1 and fused to the DNA-binding domain in pGBDUC1. Two-hybrid interactions were tested with yeast strain PJ694A, which bears the ADE2 gene as one of the reporters. Yeast cells harboring both plasmids were patched on control plates (SC–Ura–Leu) as well as experimental plates (SC–Ura–Leu–Ade) to test for protein–protein interactions in the absence or in the presence of 8 μm B02 (as indicated at the bottom).